Remarkable Configurational Stability of Magnesiated Nitriles†
Dr. Graeme Barker
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Search for more papers by this authorMadeha R. Alshawish
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Search for more papers by this authorMelanie C. Skilbeck
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Search for more papers by this authorCorresponding Author
Prof. Iain Coldham
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)Search for more papers by this authorDr. Graeme Barker
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Search for more papers by this authorMadeha R. Alshawish
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Search for more papers by this authorMelanie C. Skilbeck
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Search for more papers by this authorCorresponding Author
Prof. Iain Coldham
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)
Department of Chemistry, University of Sheffield, Sheffield, S3 7HF (UK)Search for more papers by this authorWe would like to acknowledge The Leverhulme Trust, the Libyan Ministry of Higher Education, and the University of Sheffield for funding. We are grateful to Harry Adams for the single-crystal X-ray analysis.
Graphical Abstract
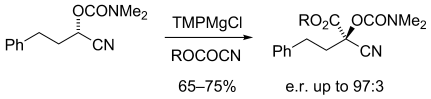
Quaternary stereocenters: Chiral α-magnesiated nitriles can be formed by deprotonation and are configurationally stable at low temperature, even for acyclic examples. These can be trapped with electrophiles to give enantiomerically enriched quaternary substituted products (see scheme; TMP=2,2,6,6-tetramethylpiperidine).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303442_sm_miscellaneous_information.pdf8.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. F. Fleming, Nat. Prod. Rep. 1999, 16, 597;
- 1bF. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk, B. C. Shook, J. Med. Chem. 2010, 53, 7902.
- 2
- 2aI. Coldham, A. J. M. Burrell, L. E. White, H. Adams, N. Oram, Angew. Chem. 2007, 119, 6271;
10.1002/ange.200701943 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6159;
- 2bA. J. M. Burrell, I. Coldham, L. Watson, N. Oram, C. D. Pilgram, N. G. Martin, J. Org. Chem. 2009, 74, 2290;
- 2cA. J. M. Burrell, I. Coldham, N. Oram, Org. Lett. 2009, 11, 1515;
- 2dA. J. M. Burrell, L. Watson, N. G. Martin, N. Oram, I. Coldham, Org. Biomol. Chem. 2010, 8, 4530;
- 2eI. Coldham, L. Watson, H. Adams, N. G. Martin, J. Org. Chem. 2011, 76, 2360;
- 2fI. Coldham, A. J. M. Burrell, L. Watson, N. Oram, N. G. Martin, Heterocycles 2012, 84, 597;
- 2gI. Coldham, A. J. M. Burrell, H. D. S. Guerrand, L. Watson, N. G. Martin, N. Oram, Beilstein J. Org. Chem. 2012, 8, 107.
- 3For chiral ligand-mediated reactions to give enantiomerically enriched α-substituted nitriles see, for example,
- 3aA. H. Mermerian, G. C. Fu, Angew. Chem. 2005, 117, 971;
10.1002/ange.200461886 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 949;
- 3bS. E. Denmark, T. W. Wilson, M. T. Burk, J. R. Heemstra, Jr., J. Am. Chem. Soc. 2007, 129, 14864;
- 3cS. E. Denmark, T. W. Wilson, Angew. Chem. 2012, 124, 10120;
10.1002/ange.201202139 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9980;
- 3dS. E. Denmark, T. W. Wilson, Synlett 2010, 1723;
- 3eJ. M. Baxter Vu, J. L. Leighton, Org. Lett. 2011, 13, 4056;
- 3fJ. N. Zhao, X. H. Liu, W. W. Luo, M. S. Xie, L. L. Lin, X. M. Feng, Angew. Chem. 2013, 125, 3557; Angew. Chem. Int. Ed. 2013, 52, 3473;
- 3gR. Kuwano, H. Miyazaki, Y. Ito, Chem. Commun. 1998, 71;
- 3hJ. Aydin, C. S. Conrad, K. J. Szabó, Org. Lett. 2008, 10, 5175;
- 3iY. Hasegawa, M. Watanabe, I. D. Gridnev, T. Ikariya, J. Am. Chem. Soc. 2008, 130, 2158;
- 3jS. Jautze, R. Peters, Angew. Chem. 2008, 120, 9424; Angew. Chem. Int. Ed. 2008, 47, 9284;
- 3kK. Hyodo, S. Nakamura, K. Tsuji, T. Ogawa, Y. Funahashi, N. Shibata, Adv. Synth. Catal. 2011, 353, 3385;
- 3lY. Kawato, N. Takahashi, N. Kumagai, M. Shibasaki, Org. Lett. 2010, 12, 1484;
- 3mB. M. Trost, J. R. Miller, C. M. Hoffman, J. Am. Chem. Soc. 2011, 133, 8165;
- 3nL. Yin, M. Kanai, M. Shibasaki, Tetrahedron 2012, 68, 3497;
- 3oH. Li, J. Song, L. Deng, Tetrahedron 2009, 65, 3139;
- 3pK. Nagata, D. Sano, Y. Shimizu, M. Miyazaki, T. Kanemitsu, T. Itoh, Tetrahedron: Asymmetry 2009, 20, 2530;
- 3qS. Shirakawa, K. Liu, H. Ito, T. N. Le, K. Maruoka, Adv. Synth. Catal. 2011, 353, 2614;
- 3rP. Chen, X. Bao, L.-F. Zhang, M. Ding, X.-J. Han, J. Li, G.-B. Zhang, Y. Q. Tu, C.-A. Fan, Angew. Chem. 2011, 123, 8311; Angew. Chem. Int. Ed. 2011, 50, 8161;
- 3sK. Ohmatsu, A. Goto, T. Ooi, Chem. Commun. 2012, 48, 7913;
- 3tH. J. Lee, S. B. Woo, D. Y. Kim, Tetrahedron Lett. 2012, 53, 3374;
- 3uL. Yin, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 9610.
- 4For other methods (chiral auxiliary, chiral electrophile, internal induction) to access enantiomerically enriched α-substituted nitriles see, for example,
- 4aJ. L. García Ruano, A. M. Martín-Castro, F. Tato, I. Alonso, J. Org. Chem. 2007, 72, 5994;
- 4bJ. L. García Ruano, A. M. Martín-Castro, F. Tato, E. Torrente, A. M. Poveda, Chem. Eur. J. 2010, 16, 6317;
- 4cJ. Guin, G. Varseev, B. List, J. Am. Chem. Soc. 2013, 135, 2100;
- 4dM. Sasaki, Y. Shirakawa, M. Kawahata, K. Yamaguchi, K. Takeda, Chem. Eur. J. 2009, 15, 3363;
- 4eM. Sasaki, M. Fujiwara, Y. Kotomori, M. Kawahata, K. Yamaguchi, K. Takeda, Tetrahedron 2013, 69, 5823.
- 5
- 5aF. F. Fleming, S. Gudipati, Eur. J. Org. Chem. 2008, 5365;
- 5bF. F. Fleming, W. Liu, S. Ghosh, O. W. Steward, J. Org. Chem. 2008, 73, 2803;
- 5cF. F. Fleming, S. Gudipati, Z. Zhang, W. Liu, O. W. Steward, J. Org. Chem. 2005, 70, 3845;
- 5dF. F. Fleming, Y. Wei, W. Liu, Z. Zhang, Tetrahedron 2008, 64, 7477;
- 5eF. F. Fleming, Z. Y. Zhang, G. Q. Wei, O. W. Steward, J. Org. Chem. 2006, 71, 1430. See also
- 5fR. J. Mycka, W. T. Eckenhoff, O. W. Steward, N. Z. Barefoot, F. F. Fleming, Tetrahedron 2013, 69, 366;
- 5gF. F. Fleming, W. Liu, Eur. J. Org. Chem. 2009, 699.
- 6For structural studies, see
- 6aG. Boche, M. Marsch, K. Harms, Angew. Chem. 1986, 98, 373; Angew. Chem. Int. Ed. Engl. 1986, 25, 373;
- 6bG. Boche, K. Harms, M. Marsch, J. Am. Chem. Soc. 1988, 110, 6925;
- 6cW. Zarges, M. Marsch, K. Harms, G. Boche, Angew. Chem. 1989, 101, 1424; Angew. Chem. Int. Ed. Engl. 1989, 28, 1392;
- 6dD. Enders, J. Kirchoff, P. Gerdes, D. Mannes, G. Raabe, J. Runsink, G. Boche, M. Marsch, H. Ahlbrecht, H. Sommer, Eur. J. Org. Chem. 1998, 63;
- 6eP. R. Carlier, C. W.-S. Lo, J. Am. Chem. Soc. 2000, 122, 12819;
- 6fR. Sott, J. Granander, G. Hilmersson, J. Am. Chem. Soc. 2004, 126, 6798;
- 6gM. Purzycki, W. Liu, G. Hilmersson, F. F. Fleming, Chem. Commun. 2013, 49, 4700.
- 7
- 7aN. N. Patwardhan, M. Gao, P. R. Carlier, Chem. Eur. J. 2011, 17, 12250;
- 7bP. R. Carlier, Y. Zhang, Org. Lett. 2007, 9, 1319.
- 8E. A. A. Wallén, J. A. M. Christiaans, M. M. Forsberg, J. I. Venäläinen, P. T. Männistö, J. Gynther, J. Med. Chem. 2002, 45, 4581.
- 9For a review on the deprotonation of aminonitriles, see T. Opatz, Synthesis 2009, 1941.
- 10M. Sasaki, E. Kawanishi, Y. Shirakawa, M. Kawahata, H. Masu, K. Yamaguchi, K. Takeda, Eur. J. Org. Chem. 2008, 3061.
- 11M. Sasaki, T. Takegawa, H. Ikemoto, M. Kawahata, K. Yamaguchi, K. Takeda, Chem. Commun. 2012, 48, 2897.
- 12For examples of in situ IR spectroscopy with organometallics, see
- 12aD. J. Pippel, G. A. Weisenburger, N. C. Faibish, P. Beak, J. Am. Chem. Soc. 2001, 123, 4919;
- 12bJ. L. Rutherford, D. Hoffmann, D. B. Collum, J. Am. Chem. Soc. 2002, 124, 264;
- 12cL. Gupta, A. C. Hoepker, K. J. Singh, D. B. Collum, J. Org. Chem. 2009, 74, 2231;
- 12dD. Stead, G. Carbone, P. O’Brien, K. R. Campos, I. Coldham, A. Sanderson, J. Am. Chem. Soc. 2010, 132, 7260;
- 12eG. Barker, J. L. McGrath, A. Klapars, D. Stead, G. Zhou, K. R. Campos, P. O’Brien, J. Org. Chem. 2011, 76, 5936;
- 12fD. Lumpi, C. Wagner, M. Schöpf, E. Horkel, G. Ramer, B. Lendl, J. Fröhlich, Chem. Commun. 2012, 48, 2451;
- 12gJ. Lefranc, A. M. Fournier, G. Mingat, S. Herbert, T. Marcelli, J. Clayden, J. Am. Chem. Soc. 2012, 134, 7286;
- 12hA. M. Fournier, C. J. Nichols, M. A. Vincent, I. H. Hillier, J. Clayden, Chem. Eur. J. 2012, 18, 16478;
- 12iN. S. Sheikh, D. Leonori, G. Barker, J. D. Firth, K. R. Campos, A. J. H. M. Meijer, P. O’Brien, I. Coldham, J. Am. Chem. Soc. 2012, 134, 5300;
- 12jX. Li, D. Leonori, N. S. Sheikh, I. Coldham, Chem. Eur. J. 2013, 19, 7724.