Organocatalytic Diastereo- and Enantioselective 1,3-Dipolar Cycloaddition of Azlactones and Methyleneindolinones†
Wangsheng Sun
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
These authors contributed equally.
Search for more papers by this authorGongming Zhu
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
These authors contributed equally.
Search for more papers by this authorChongyang Wu
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Search for more papers by this authorGuofeng Li
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Search for more papers by this authorCorresponding Author
Dr. Liang Hong
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)Search for more papers by this authorCorresponding Author
Prof. Dr. Rui Wang
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)Search for more papers by this authorWangsheng Sun
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
These authors contributed equally.
Search for more papers by this authorGongming Zhu
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
These authors contributed equally.
Search for more papers by this authorChongyang Wu
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Search for more papers by this authorGuofeng Li
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Search for more papers by this authorCorresponding Author
Dr. Liang Hong
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)Search for more papers by this authorCorresponding Author
Prof. Dr. Rui Wang
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou, 730000 (P.R. China)Search for more papers by this authorWe gratefully acknowledge financial support from the NSFC (20932003, 91213302, 21272102, 21202071) and the National S&T Major Project of China (2012ZX09504001-003). W.S. is grateful for a Scholarship Award for Distinguished Doctoral Candidates granted by the Ministry of Education, China.
Graphical Abstract
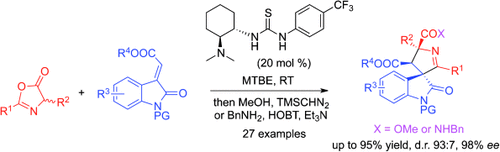
A simple route to complexity: An organocatalytic 1,3-dipolar cycloaddition between azlactones and methyleneindolinones provided spirooxindoles with high enantioselectivity (see scheme). This transformation takes advantage of the nucleophilic C4 and electrophilic C2 atoms in the azlactone substrate. Bn=benzyl, HOBt=1-hydroxy-1H-benzotriazole, MTBE=methyl tert-butyl ether, PG=protecting group, TMS=trimethylsilyl.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302831_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For seminal studies, see:
- 1aJ. Plöchl, Ber. Dtsch. Chem. Ges. 1883, 16, 2815;
10.1002/cber.188301602235 Google Scholar
- 1bE. Erlenmeyer, Ber. Dtsch. Chem. Ges. 1900, 33, 2036; for reviews, see:
- 1cJ. S. Fisk, R. A. Mosey, J. J. Tepe, Chem. Soc. Rev. 2007, 36, 1432;
- 1dN. M. Hewlett, C. D. Hupp, J. J. Tepe, Synthesis 2009, 2825;
- 1eR. A. Mosey, J. S. Fisk, J. J. Tepe, Tetrahedron: Asymmetry 2008, 19, 2755;
- 1fA.-N. R. Alba, R. Rios, Chem. Asian J. 2011, 6, 720.
- 2For selected representative examples, see:
- 2aJ. C. Ruble, G. C. Fu, J. Am. Chem. Soc. 1998, 120, 11532;
- 2bS. A. Shaw, P. Aleman, E. Vedejs, J. Am. Chem. Soc. 2003, 125, 13368;
- 2cS. A. Shaw, P. Aleman, J. Christy, J. W. Kampf, P. Va, E. Vedejs, J. Am. Chem. Soc. 2006, 128, 925;
- 2dC. Joannesse, C. P. Johnston, C. Concellón, C. Simal, D. Philp, A. D. Smith, Angew. Chem. 2009, 121, 9076;
10.1002/ange.200904333 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8914;
- 2eC. Kanta De, N. Mittal, D. Seidel, J. Am. Chem. Soc. 2011, 133, 16802;
- 2fB. M. Trost, C. Jäkel, B. Plietker, J. Am. Chem. Soc. 2003, 125, 4438;
- 2gB. M. Trost, X. Ariza, J. Am. Chem. Soc. 1999, 121, 10727;
- 2hB. M. Trost, C. B. Lee, J. Am. Chem. Soc. 1998, 120, 6818;
- 2iB. M. Trost, K. Dogra, J. Am. Chem. Soc. 2002, 124, 7256;
- 2jD. Uraguchi, Y. Asai, T. Ooi, Angew. Chem. 2009, 121, 747; Angew. Chem. Int. Ed. 2009, 48, 733;
- 2kM. Weber, S. Jautze, W. Frey, R. Peters, Chem. Eur. J. 2012, 18, 14792.
- 3For selected examples of Mannich-type reactions, see:
- 3aD. Uraguchi, Y. Ueki, T. Ooi, J. Am. Chem. Soc. 2008, 130, 14088;
- 3bD. Uraguchi, K. Koshimoto, T. Ooi, Chem. Commun. 2010, 46, 300;
- 3cX. Liu, L. Deng, X. Jiang, W. Yan, C. Liu, R. Wang, Org. Lett. 2010, 12, 876;
- 3dX. Liu, L. Deng, H. Song, H. Jia, R. Wang, Org. Lett. 2011, 13, 1494;
- 3eS.-H. Shi, F.-P. Huang, P. Zhu, Z.-W. Dong, X.-P. Hui, Org. Lett. 2012, 14, 2010;
- 3fW.-Q. Zhang, L.-F. Cheng, J. Yu, L.-Z. Gong, Angew. Chem. 2012, 124, 4161; Angew. Chem. Int. Ed. 2012, 51, 4085; for aldol-type reactions, see:
- 3gM. Terada, H. Tanaka, K. Sorimachi, J. Am. Chem. Soc. 2009, 131, 3430;
- 3hT. Misaki, G. Takimoto, T. Sugimura, J. Am. Chem. Soc. 2010, 132, 6286.
- 4
- 4aS. Cabrera, E. Reyes, J. Alemán, A. Milelli, S. Kobbelgaard, K. A. Jørgensen, J. Am. Chem. Soc. 2008, 130, 12031;
- 4bJ. Alemán, A. Milelli, S. Cabrera, E. Reyes, K. A. Jørgensen, Chem. Eur. J. 2008, 14, 10958;
- 4cH. Jiang, M. W. Paixão, D. Monge, K. A. Jørgensen, J. Am. Chem. Soc. 2010, 132, 2775;
- 4dD. Uraguchi, Y. Ueki, T. Ooi, Science 2009, 326, 120;
- 4eA.-N. R. Alba, G. Valero, T. Calbet, M. Font-Bardía, A. Moyano, R. Rios, Chem. Eur. J. 2010, 16, 9884;
- 4fA.-N. R. Alba, X. Companyó, G. Valero, A. Moyano, R. Rios, Chem. Eur. J. 2010, 16, 5354;
- 4gD. Uraguchi, Y. Ueki, A. Sugiyama, T. Ooi, Chem. Sci. 2013, 4, 1308.
- 5
- 5aS. Dong, X. Liu, X. Chen, F. Mei, Y. Zhang, B. Gao, L. Lin, X. Feng, J. Am. Chem. Soc. 2010, 132, 10650;
- 5bS. Dong, X. Liu, Y. Zhang, L. Lin, X. Feng, Org. Lett. 2011, 13, 5060;
- 5cJ. Jiang, J. Qing, L.-Z. Gong, Chem. Eur. J. 2009, 15, 7031;
- 5dM. Terada, H. Nii, Chem. Eur. J. 2011, 17, 1760;
- 5eY. Ying, Z. Chai, H.-F. Wang, P. Li, C.-W. Zheng, G. Zhao, Y.-P. Cai, Tetrahedron 2011, 67, 3337;
- 5fX. Jiang, H. Zhu, X. Shi, Y. Zhong, Y. Li, R. Wang, Adv. Synth. Catal. 2013, 355, 308.
- 6For racemic transformations, see:
- 6aV. Sharma, J. J. Tepe, Org. Lett. 2005, 7, 5091;
- 6bR. S. Z. Saleem, J. J. Tepe, J. Org. Chem. 2010, 75, 4330;
- 6cS. Peddibhotla, J. J. Tepe, Synthesis 2003, 1433;
- 6dS. Peddibhotla, J. J. Tepe, J. Am. Chem. Soc. 2004, 126, 12776.
- 7For reviews of 1,3-dipolar cycloaddition, see:
- 7a 1,3-Dipolar Cycloaddition Chemistry (Ed.: ), Wiley, New York, 1984;
- 7bA. Padwa, W. Pearson, Synthetic Applications of Dipolar Cycloaddition Chemistry towards Heterocyclic and Natural Products, Wiley-VCH, Weinheim, 2002;
10.1002/0471221902 Google Scholar
- 7cL. M. Stanley, M. P. Sibi, Chem. Rev. 2008, 108, 2887;
- 7dK. V. Gothelf, K. A. Jørgensen, Chem. Rev. 1998, 98, 863;
- 7eG. Pandey, P. Banerjee, S. R. Gadre, Chem. Rev. 2006, 106, 4484.
- 8
- 8aA. D. Melhado, M. Luparia, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 12638;
- 8bA. D. Melhado, G. W. Amarante, Z. J. Wang, M. Luparia, F. D. Toste, J. Am. Chem. Soc. 2011, 133, 3517;
- 8cM. Martín-Rodríguez, C. Nájera, J. M. Sansano, Synlett 2012, 62.
- 9
- 9aC. V. Galliford, K. A. Scheidt, Angew. Chem. 2007, 119, 8902;
10.1002/ange.200701342 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8748;
- 9bC. Marti, E. M. Carreira, Eur. J. Org. Chem. 2003, 2209;
- 9cH. Lin, S. J. Danishefsky, Angew. Chem. 2003, 115, 38; Angew. Chem. Int. Ed. 2003, 42, 36;
- 9dK. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps, S. Wang, J. Am. Chem. Soc. 2005, 127, 10130;
- 9eS. Shangary, D. Qin, D. McEachern, M. Liu, R. S. Miller, S. Qiu, Z. Nikolovska-Coleska, K. Ding, G. Wang, J. Chen, D. Bernard, J. Zhang, Y. Lu, Q. Gu, R. B. Shah, K. J. Pienta, X. Ling, S. Kang, M. Guo, Y. Sun, D. Yang, S. Wang, Proc. Natl. Acad. Sci. USA 2008, 105, 3933.
- 10For reviews, see:
- 10aB. M. Trost, M. K. Brennan, Synthesis 2009, 3003;
- 10bG. S. Singh, Z. Y. Desta, Chem. Rev. 2012, 112, 6104;
- 10cR. Dalpozzo, G. Bartoli, G. Bencivenni, Chem. Soc. Rev. 2012, 41, 7247;
- 10dN. R. Ball-Jones, J. J. Badillo, A. K. Franz, Org. Biomol. Chem. 2012, 10, 5165;
- 10eF. Zhou, Y.-L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381;
- 10fL. Hong, R. Wang, Adv. Synth. Catal. 2013, 355, 1023.
- 11
- 11aA. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh, H. Waldmann, Nat. Chem. 2010, 2, 735;
- 11bX.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao, L.-Z. Gong, J. Am. Chem. Soc. 2009, 131, 13819;
- 11cF. Shi, Z.-L. Tao, S.-W. Luo, S.-J. Tu, L.-Z. Gong, Chem. Eur. J. 2012, 18, 6885;
- 11dL.-L. Wang, J.-F. Bai, L. Peng, L.-W. Qi, L.-N. Jia, Y.-L. Guo, X.-Y. Luo, X.-Y. Xu, L.-X. Wang, Chem. Commun. 2012, 48, 5175;
- 11eB. Tan, X. Zeng, W. W. Y. Leong, Z. Shi, C. F. Barbas III, G. Zhong, Chem. Eur. J. 2012, 18, 63.
- 12
- 12aX. Jiang, Y. Cao, Y. Wang, L. Liu, F. Shen, R. Wang, J. Am. Chem. Soc. 2010, 132, 15328;
- 12bY. Cao, X. Jiang, L. Liu, F. Shen, F. Zhang, R. Wang, Angew. Chem. 2011, 123, 9290; Angew. Chem. Int. Ed. 2011, 50, 9124;
- 12cW. Sun, G. Zhu, C. Wu, L. Hong, R. Wang, Chem. Eur. J. 2012, 18, 6737;
- 12dX. Jiang, Y. Sun, J. Yao, Y. Cao, M. Kai, N. He, X. Zhang, Y. Wang, R. Wang, Adv. Synth. Catal. 2012, 354, 917;
- 12eW. Sun, G. Zhu, C. Wu, L. Hong, R. Wang, Chem. Eur. J. 2012, 18, 13959;
- 12fY.-M. Cao, F.-F. Shen, F.-T. Zhang, R. Wang, Chem. Eur. J. 2013, 19, 1184;
- 12gQ. Chen, J. Liang, S. Wang, D. Wang, R. Wang, Chem. Commun. 2013, 49, 1657;
- 12hL. Hong, M. Kai, C. Wu, W. Sun, G. Zhu, G. Li, X. Yao, R. Wang, Chem. Commun. 2013, DOI: .
- 13CCDC 932360 (5 b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.