Rational Design of an Apoptosis-Inducing Photoreactive DNA Intercalator†
Nico Ueberschaar
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Search for more papers by this authorDr. Hans-Martin Dahse
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Search for more papers by this authorTom Bretschneider
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christian Hertweck
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Friedrich Schiller University, Jena (Germany)
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)Search for more papers by this authorNico Ueberschaar
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Search for more papers by this authorDr. Hans-Martin Dahse
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Search for more papers by this authorTom Bretschneider
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christian Hertweck
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)
Friedrich Schiller University, Jena (Germany)
Leibniz Institute for Natural Product Research and Infection Biology, HKI, Beutenbergstr. 11a, 07745 Jena (Germany)Search for more papers by this authorWe thank A. Perner for MS analysis, H. Heinecke for NMR measurements, T. Palenta for assistance in synthesis and E.-M. Neumann for assistance in biological assays. This work was supported by the Federal Ministry of Science and Technology (BMBF (Germany)).
Graphical Abstract
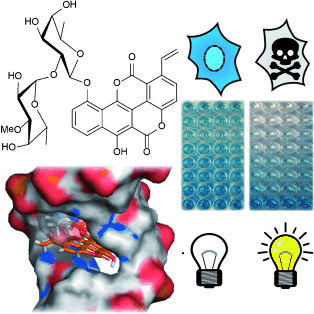
Light on DNA intercalators: Molecular modeling and mutasynthesis were employed to rationally tailor the antitumoral agent chartreusin into a vinyl-substituted derivative. Exposure with visible light dramatically improved antiproliferative activities owing to covalent binding with DNA and induction of apoptosis. The results hold promise for a more efficient chemotherapy, in particular for selectively treating tumors with light probes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302439_sm_miscellaneous_information.pdf2.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward, D. Forman, CA-Cancer J. Clin. 2011, 61, 69–90.
- 2P. G. Grothaus, G. M. Cragg, D. J. Newman, Curr. Org. Chem. 2010, 14, 1781–1791.
- 3A. C. Souza, A. de Fatima, R. B. da Silveira, G. Z. Justo, Curr. Drug Targets 2012, 13, 1072–1082.
- 4
- 4aJ. Portugal, Curr. Med. Chem. 2003, 3, 411–420;
- 4bM. Uramoto, T. Kusano, T. Nishio, K. Isono, K. Shishido, T. Ando, Febs Lett. 1983, 153, 325–328.
- 5A. Lorico, B. H. Long, Eur. J. Cancer 1993, 29A, 1985–1991.
- 6
- 6aT. Tashiro, K. Kon, M. Yamamoto, N. Yamada, T. Tsuruo, S. Tsukagoshi, Cancer Chemother. Pharmacol. 1994, 34, 287–292;
- 6bJ. P. McGovren, G. L. Neil, S. L. Crampton, M. I. Robinson, J. D. Douros, Cancer Res. 1977, 37, 1666–1672.
- 7W. M. Sharman, C. M. Allen, J. E. van Lier, Drug Discovery Today 1999, 4, 507–517.
- 8J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue, T. Hasan, Chem. Rev. 2010, 110, 2795–2838.
- 9F. E. Fox, Z. Niu, A. Tobia, A. H. Rook, J. Invest. Dermatol. 1998, 111, 327–332.
- 10S. Ibsen, E. Zahavy, W. Wrasdilo, M. Berns, M. Chan, S. Esener, Pharm. Res. 2010, 27, 1848–1860.
- 11
- 11aJ. Ramos, J. Villa, A. Ruiz, R. Armstrong, J. Matta, Cancer Epidemiol. Biomarkers Prev. 2004, 13, 2006–2011;
- 11bS. Hu, F. C. Ma, F. Collado-Mesa, R. S. Kirsner, Arch. Dermatol. 2004, 140, 819–824.
- 12
- 12aB. S. Howerton, D. K. Heidary, E. C. Glazer, J. Am. Chem. Soc. 2012, 134, 8324–8327;
- 12bS. J. Berners-Price, Angew. Chem. 2011, 123, 830–831; Angew. Chem. Int. Ed. 2011, 50, 804–805.
- 13
- 13aX. Salas, J. Portugal, Febs Lett. 1991, 292, 223–228;
- 13bP. L. Hamilton, D. P. Arya, Nat. Prod. Rep. 2012, 29, 134–143.
- 14C. G. Ricci, P. A. Netz, J. Chem. Inf. Model. 2009, 49, 1925–1935.
- 15
- 15aL. R. McGee, R. Misra, J. Am. Chem. Soc. 1990, 112, 2386–2389;
- 15bY. Q. Li, X. S. Huang, K. Ishida, A. Maier, G. Kelter, Y. Jiang, G. Peschel, K. D. Menzel, M. G. Li, M. L. Wen, L. H. Xu, S. Grabley, H. H. Fiebig, C. L. Jiang, C. Hertweck, I. Sattler, Org. Biomol. Chem. 2008, 6, 3601–3605;
- 15cM. Greenstein, T. Monji, R. Yeung, W. M. Maiese, R. J. White, Antimicrob. Agents Chemother. 1986, 29, 861–866;
- 15dR. K. Elespuru, S. K. Gonda, Science 1984, 223, 69–71.
- 16
- 16aJ. Kennedy, Nat. Prod. Rep. 2008, 25, 25–34;
- 16bS. Weist, R. D. Sussmuth, Appl. Microbiol. Biotechnol. 2005, 68, 141–150;
- 16cA. Kirschning, F. Hahn, Angew. Chem. 2012, 124, 4086–4096;
10.1002/ange.201107386 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4012–4022.
- 17Z. Xu, K. Jakobi, K. Welzel, C. Hertweck, Chem. Biol. 2005, 12, 579–588.
- 18C. Hertweck, A. Luzhetskyy, Y. Rebets, A. Bechthold, Nat. Prod. Rep. 2007, 24, 162–190.
- 19Z. Xu, PhD thesis, Friedrich Schiller University Jena, 2008.
- 20D. Mal, A. Patra, H. Roy, Tetrahedron Lett. 2004, 45, 7895–7898.
- 21
- 21aM. S. Malamas, E. S. Manas, R. E. McDevitt, I. Gunawan, Z. B. Xu, M. D. Collini, C. P. Miller, T. Dinh, R. A. Henderson, J. C. Keith, H. A. Harris, Curr. Med. Chem. 2004, 47, 5021–5040;
- 21bF. Shibahara, K. Nozaki, T. Matsuo, T. Hiyama, Bioorg. Med. Chem. Lett. 2002, 12, 1825–1827.
- 22
- 22aM. Ziehl, J. He, H.-M. Dahse, C. Hertweck, Angew. Chem. 2005, 117, 1226–1230;
10.1002/ange.200461990 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1202–1205;
- 22bM. Werneburg, B. Busch, J. He, M. E. A. Richter, L. K. Xiang, B. S. Moore, M. Roth, H. M. Dahse, C. Hertweck, J. Am. Chem. Soc. 2010, 132, 10407–10413;
- 22cA. S. Eustáquio, B. S. Moore, Angew. Chem. 2008, 120, 4000–4002;
10.1002/ange.200800177 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3936–3938;
- 22dS. Eichner, T. Knobloch, H. G. Floss, J. Fohrer, K. Harmrolfs, J. Hermane, A. Schulz, F. Sasse, P. Spiteller, F. Taft, A. Kirschning, Angew. Chem. 2012, 124, 776–781;
10.1002/ange.201106249 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 752–757;
- 22eT. Knobloch, K. Harmrolfs, F. Taft, B. Thomaszewski, F. Sasse, A. Kirschning, ChemBioChem 2011, 12, 540–547;
- 22fF. Taft, K. Harmrolfs, I. Nickeleit, A. Heutling, M. Kiene, N. Malek, F. Sasse, A. Kirschning, Chem. Eur. J. 2012, 18, 880–886.