Silver-Mediated Cycloaddition of Alkynes with CF3CHN2: Highly Regioselective Synthesis of 3-Trifluoromethylpyrazoles†
Feng Li
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorJing Nie
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorLong Sun
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorYan Zheng
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorCorresponding Author
Prof. Jun-An Ma
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)Search for more papers by this authorFeng Li
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorJing Nie
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorLong Sun
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorYan Zheng
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorCorresponding Author
Prof. Jun-An Ma
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)
Department of Chemistry, Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072 (China)Search for more papers by this authorThis work was supported financially by the NSFC (No. 21002068, 21172170, and 21225208). We also thank Dr. Dominique Cahard (CNRS & Université et INSA de Rouen, France) for his helpful discussions and suggestions.
Graphical Abstract
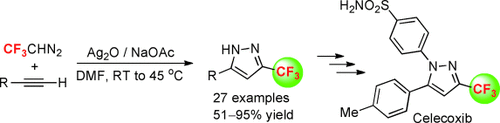
Silver screen: The title reaction provides a convenient and efficient method for the construction of 5-substituted 3-trifluoromethylpyrazoles under mild reaction conditions. By using this protocol, the marketed drug Celecoxib (antiarthritic) could be easily synthesized (see scheme; DMF=N,N-dimethylformamide).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301870_sm_miscellaneous_information.pdf941.3 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Lamberth, Heterocycles 2007, 71, 1467;
- 1bJ. Elguero, A. M. S. Silva, A. C. Tomé, Modern Heterocyclic Chemistry, 1st ed., Wiley-VCH, Weinheim, 2011, p. 635.
- 2
- 2aT. D. Penning, J. J. Talley, S. R. Bertenshaw, J. S. Carter, P. W. Collins, S. Doctor, M. J. Greveto, L. F. Lee, J. W. Malecha, J. M. Miyashiro, R. S. Rogers, D. J. Rogier, S. S. Yu, G. D. Anderson, E. G. Burton, S. A. Gregory, C. M. Icoboldt, W. E. Perkus, K. Seibert, A. W. Veenhuizen, Y. Y. Zhang, P. C. Isakson, J. Med. Chem. 1997, 40, 1347 (Celecoxib);
- 2bS. R. Cox, S. P. Lesman, J. F. Boucher, M. J. Krautmann, B. D. Hummel, M. Savides, S. Marsh, A. Fielder, M. R. Stegemann, J. Vet. Pharmacol. Ther. 2010, 33, 461 (Mavacoxib);
- 2cE. Lee, M. K. Choi, H. J. Youk, C. H. Kim, I. C. Han, B. C. Yoo, M. K. Lee, S. J. Lim, J. Cancer Res. Clin. Oncol. 2006, 132, 232 (SC-560);
- 2dA. Sun, N. Chandrakumar, J.-J. Yoon, R. K. Plemper, J. P. Snyder, Bioorg. Med. Chem. Lett. 2007, 17, 5199 (AS-136A);
- 2eJ. G. Varnes, D. A. Wacker, D. J. P. Pinto, M. J. Orwat, J. P. Theroff, B. Wells, R. A. Galemo, J. M. Luettgen, R. M. Knabb, S. Bai, K. He, P. Y. S. Lam, R. R. Wexler, Bioorg. Med. Chem. Lett. 2008, 18, 749 (Razaxaban);
- 2fG. P. Lahm, T. P. Selby, J. H. Freudenberger, T. M. Stevenson, B. J. Myers, G. Seburyamo, B. K. Smith, L. Flexner, C. E. Clark, D. Cordova, Bioorg. Med. Chem. Lett. 2005, 15, 4898 (DP-23).
- 3
- 3aL. Yet, Comprehensive heterocyclic chemistry III, 1st ed., Elsevier, Oxford, 2008, 4, 1;
- 3bS. Fustero, M. Sánchez-Roselló, P. Barrio, A. Simón-Fuentes, Chem. Rev. 2011, 111, 6984.
- 4Selected recent examples, see:
- 4aT. Norris, R. Colon-Cruz, D. H. B. Ripin, Org. Biomol. Chem. 2005, 3, 1844;
- 4bA. Shavnya, S. M. Sakya, M. L. Munich, B. Rast, K. L. DeMello, B. H. Jaynes, Tetrahedron Lett. 2005, 46, 6887;
- 4cS. P. Singh, V. Kumar, R. Aggarwal, J. Elguero, J. Heterocycl. Chem. 2006, 43, 1;
- 4dP. S. Humphries, J. M. Finefield, Tetrahedron Lett. 2006, 47, 2443;
- 4eY. Yonetoku, H. Kubota, Y. Okamoto, A. Toyoshima, M. Funatsu, J. Ishikawa, M. Takeuchi, M. Ohta, S.-I. Tsukamoto, Bioorg. Med. Chem. 2006, 14, 4750;
- 4fV. Montoya, J. Pons, J. García-Antón, X. Solans, M. Font-Bardia, J. Ros, J. Fluorine Chem. 2007, 128, 1007;
- 4gS. Fustero, R. Román, J. F. Sanz-Cervera, A. Simón-Fuentes, A. C. Cuñat, S. Villanova, M. Murguía, J. Org. Chem. 2008, 73, 3523;
- 4hH. Dai, Y.-Q. Li, D. Du, X. Qin, X. Zhang, H.-B. Yu, J.-X. Fang, J. Agric. Food Chem. 2008, 56, 10805;
- 4iX. Yang, S. Shui, X. Chen, H. He, F. Wu, J. Fluorine Chem. 2010, 131, 426.
- 5
- 5aP. Le Maux, S. Juillard, G. Simmoneaux, Synthesis 2006, 1701;
- 5bP. K. Mykhailiuk, S. Afonin, G. V. Palamarchuk, O. V. Shishkin, A. S. Ulrich, I. V. Komarov, Synthesis 2008, 1757;
- 5cP. K. Mykhailiuk, S. Afonin, G. V. Palamarchuk, O. V. Shishkin, A. S. Ulrich, I. V. Komarov, Angew. Chem. 2008, 120, 5849; Angew. Chem. Int. Ed. 2008, 47, 5765;
- 5dO. O. Grygorenko, O. S. Artamonov, I. V. Komarov, P. K. Mykhailiuk, Tetrahedron 2011, 67, 803.
- 6aB. Morandi, E. M. Carreira, Angew. Chem. 2010, 122, 950;
10.1002/ange.200905573 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 938;
- 6bB. Morandi, E. M. Carreira, Angew. Chem. 2010, 122, 4390;
10.1002/ange.201000787 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4294;
- 6cB. Morandi, B. Mariampillai, E. M. Carreira, Angew. Chem. 2011, 123, 1133; Angew. Chem. Int. Ed. 2011, 50, 1101;
- 6dB. Morandi, E. M. Carreira, Angew. Chem. 2011, 123, 9251;
10.1002/ange.201103526 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9085;
- 6eB. Morandi, J. Cheang, E. M. Carreira, Org. Lett. 2011, 13, 3080.
- 7C.-B. Liu, W. Meng, F. Li, S. Wang, J. Nie, J.-A. Ma, Angew. Chem. 2012, 124, 6331; Angew. Chem. Int. Ed. 2012, 51, 6227.
- 8Z. Chai, J.-P. Bouillon, D. Cahard, Chem. Commun. 2012, 48, 9471.
- 9R. Fields, J. P. Tomlinson, J. Fluorine Chem. 1979, 13, 147.
- 10aN. Jiang, C.-J. Li, Chem. Commun. 2004, 394;
- 10bX. Qi, J. M. Ready, Angew. Chem. 2007, 119, 3306; Angew. Chem. Int. Ed. 2007, 46, 3242;
- 10cS. He, L. Chen, Y.-N. Niu, L.-Y. Wu, Y.-M. Liang, Tetrahedron Lett. 2009, 50, 2443;
- 10dD. Vuluga, J. Legros, B. Crousse, D. Bonnet-Delpon, Green Chem. 2009, 11, 156;
- 10eL. Lin, J. Zhang, R. Wang, Asian J. Org. Chem. 2012, 1, 222.
- 11CCDC 889113 (2 b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12A. Vitérisi, A. Orsini, J.-M. Weibel, P. Pale, Tetrahedron Lett. 2006, 47, 2779.