Reversible σ-Dimerizations of Persistent Organic Radical Cations†
Xiaoyu Chen
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorDr. Xingyong Wang
Theoretical and Computational Chemistry Institute, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorZhaoyi Zhou
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorProf. Yizhi Li
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorYunxia Sui
Centre of Modern Analysis, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorProf. Jing Ma
Theoretical and Computational Chemistry Institute, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorCorresponding Author
Prof. Xinping Wang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Xinping Wang, State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Philip P. Power, Department of Chemistry, University of California, Davis, CA 95616 (USA)
Search for more papers by this authorCorresponding Author
Prof. Philip P. Power
Department of Chemistry, University of California, Davis, CA 95616 (USA)
Xinping Wang, State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Philip P. Power, Department of Chemistry, University of California, Davis, CA 95616 (USA)
Search for more papers by this authorXiaoyu Chen
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorDr. Xingyong Wang
Theoretical and Computational Chemistry Institute, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorZhaoyi Zhou
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorProf. Yizhi Li
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorYunxia Sui
Centre of Modern Analysis, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorProf. Jing Ma
Theoretical and Computational Chemistry Institute, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Search for more papers by this authorCorresponding Author
Prof. Xinping Wang
State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Xinping Wang, State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Philip P. Power, Department of Chemistry, University of California, Davis, CA 95616 (USA)
Search for more papers by this authorCorresponding Author
Prof. Philip P. Power
Department of Chemistry, University of California, Davis, CA 95616 (USA)
Xinping Wang, State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (China)
Philip P. Power, Department of Chemistry, University of California, Davis, CA 95616 (USA)
Search for more papers by this authorWe thank the US National Science Foundation (CHE-0948417, P.P.P.), the National Natural Science Foundation of China (Grants 21171087, 91122019, 21021062, X.W. and 20825312, J.M.), and the Natural Science Foundation of Jiangsu Province (Grant BK2011549, X.W.) for financial support. We are grateful to the High Performance Computing Centre of Nanjing University for providing the IBM Blade cluster system. Part of the computational work has been done on the Sugon TC5000 high-performance linux cluster at ITCC.
Graphical Abstract
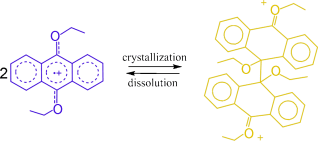
A class of well-defined reversible σ-dimerizations of 9,10-dialkoxyanthracene radical cations are presented. Yellow crystals of the σ-dimerized dication dissociate to purple solutions of monomeric radical cations in solution (see scheme). The identity and stability of radical cations were unequivocally confirmed, providing evidence for reversible σ-dimerizations of persistent radical cations of aromatic systems.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201207412_sm_miscellaneous_information.pdf723.5 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Schmittel, A. Burghart, Angew. Chem. 1997, 109, 2658–2699;
10.1002/ange.19971092304 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2550–2589;
- 1bA. A. O. Sarhan, C. Bolm, Chem. Soc. Rev. 2009, 38, 2730–2744.
- 2
- 2aJ. Heinze, B. A. Frontana-Uribe, S. Ludwigs, Chem. Rev. 2010, 110, 4724–4771;
- 2bH. Higashimura, S. Kobayashi in Encyclopedia of Polymer Science and Technology (Eds.: ), Wiley, New York, 2004;
- 2cA. F. Diaz in Organic Electrochemistry (Eds.: ), Wiley, New York, 1991.
- 3
- 3aA. Smie, J. Heinze, Angew. Chem. 1997, 109, 375–379;
10.1002/ange.19971090413 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 363–367;
- 3bA. Merz, J. Kronberger, L. Dunsch, A. Neudeck, A. Petr, L. Parkanyi, Angew. Chem. 1999, 111, 1533–1538;
10.1002/(SICI)1521-3757(19990517)111:10<1533::AID-ANGE1533>3.0.CO;2-W Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1442–1446;10.1002/(SICI)1521-3773(19990517)38:10<1442::AID-ANIE1442>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 3cR. Rathore, P. L. Magueres, S. V. Lindeman, J. K. Kochi, Angew. Chem. 2000, 112, 818–821;
10.1002/(SICI)1521-3757(20000218)112:4<818::AID-ANGE818>3.0.CO;2-# Google ScholarAngew. Chem. Int. Ed. 2000, 39, 809–812;10.1002/(SICI)1521-3773(20000218)39:4<809::AID-ANIE809>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 3dJ. Heinze, C. Willmann, P. Bäuerle, Angew. Chem. 2001, 113, 2936–2940;
10.1002/1521-3757(20010803)113:15<2936::AID-ANGE2936>3.0.CO;2-3 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2861–2864;10.1002/1521-3773(20010803)40:15<2861::AID-ANIE2861>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 3eP. Tschuncky, J. Heinze, M. M. Ghoneim, Electrochem. Commun. 2001, 3, 697–702;
- 3fO. Yurchenko, D. Freytag, L. Borg, R. Zentel, J. Heinze, L. Sabine, J. Phys. Chem. B 2012, 116, 30–39.
- 4
- 4aF. Effenberger, W. D. Stohrer, A. Steinbach, Angew. Chem. 1969, 81, 1046–1047;
10.1002/ange.19690812408 Google ScholarAngew. Chem. Int. Ed. Engl. 1969, 8, 280–281;
- 4bF. Effenberger, K. Mack, R. Niess, F. Reisinger, A. Steinbach, W. Stohrer, J. J. Stezowski, I. Rommel, A. Maier, J. Org. Chem. 1988, 53, 4379–4386;
- 4cF. Effenberger, Acc. Chem. Res. 1989, 22, 27–35;
- 4dF. Effenberger, W. Stohrer, K. Mack, F. Reisinger, W. Seufert, H. E. A. Kramer, R. Főll, E. Vogelmann, J. Am. Chem. Soc. 1990, 112, 4849–4857.
- 5
- 5aT. Ramnial, I. McKenzie, B. Gorodetsky, E. M. W. Tsang, J. A. C. Clyburne, J. Chem. Soc. Chem. Commun. 2004, 1054–1055;
- 5bJ. Taquet, O. Siri, J. Collin, A. Messaoudi, P. Braunstein, New J. Chem. 2005, 29, 188–192.
- 6The persistent radical cation is defined as a species that is sufficiently long-lived to be observed by conventional spectroscopic methods. This restriction requires a half-life of the order of minutes:
- 6aD. Griller, K. U. Ingold, Acc. Chem. Res. 1976, 9, 13–19;
- 6bP. P. Power, Chem. Rev. 2003, 103, 789–809;
- 6cR. G. Hicks, Org. Biomol. Chem. 2007, 5, 1321–1338.
- 7Selected examples:
- 7aD. D. Graf, J. P. Campbell, L. L. Miller, K. R. Mann, J. Am. Chem. Soc. 1996, 118, 5480–5481;
- 7bD. Yamazaki, T. Nishinaga, N. Tanino, K. Komatsu, J. Am. Chem. Soc. 2006, 128, 14470–14471;
- 7cM. Banerjee, S. V. Lindeman, R. Rathore, J. Am. Chem. Soc. 2007, 129, 8070–8071.
- 8For more examples, see:
- 8aL. L. Miller, K. R. Mann, Acc. Chem. Res. 1996, 29, 417–423;
- 8bT. Nishinaga, K. Komatsu, Org. Biomol. Chem. 2005, 3, 561–569, and references therein.
- 9aI. Krossing, I. Raabe, Angew. Chem. 2004, 116, 2116–2142; Angew. Chem. Int. Ed. 2004, 43, 2066–2090, and references therein;
- 9bA. Decken, J. Passmore, X. Wang, Angew. Chem. 2006, 118, 2839–2843;
10.1002/ange.200504262 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2773–2777;
- 9cX. Chen, B. Ma, X. Wang, S. Yao, L. Ni, Z. Zhou, Y. Li, W. Huang, J. Ma, J. Zuo, X. Wang, Chem. Eur. J. 2012, 18, 11828–11836.
- 10
- 10aX.-Z. Zhu, C.-F. Chen, J. Am. Chem. Soc. 2005, 127, 13158–13159;
- 10bX.-Z. Zhu, C.-F. Chen, J. Org. Chem. 2005, 70, 917–924;
- 10cH. Okii, H. Hara, Y. Ohba, Jpn. J. Appl. Phys. 1992, 31, L416–L418;
- 10dD. J. Fatemi, H. Murata, C. D. Merritt, Z. H. Kafafi, Synth. Met. 1997, 85, 1225–1228;
- 10eM. Yu, J. Duan, C. Lin, C. Cheng, Y. Tao, Chem. Mater. 2002, 14, 3958–3963;
- 10fJ. R. Quinn, F. W. Foss, Jr., L. Venkataraman, M. S. Hybertsen, R. Breslow, J. Am. Chem. Soc. 2007, 129, 6714–6715;
- 10gJ. R. Quinn, F. W. Foss, Jr., L. Venkataraman, R. Breslow, J. Am. Chem. Soc. 2007, 129, 12376–12377.
- 11M. Bendikov, F. Wudl, D. Perepichka, Chem. Rev. 2004, 104, 4891–4945, and references therein.
- 12aA. Matsuura, T. Nishinaga, K. Komatsu, J. Am. Chem. Soc. 2000, 122, 10007–10016;
- 12bM. J. Modjewski, R. Shukla, S. V. Lindeman, R. Rathore, Tetrahedron Lett. 2009, 50, 6687–6690;
- 12cR. Sebastiano, J. D. Korp, J. K. Kochi, J. Chem. Soc. Chem. Commun. 1991, 1481–1482;
- 12dS. R. Belding, N. V. Rees, L. Aldous, C. Hardacre, R. G. Compton, J. Phys. Chem. C 2008, 112, 1650–1657.
- 13J. S. Meek, P. A. Monroe, C. J. Bouboulis, J. Org. Chem. 1963, 28, 2572–2577.
- 14M. P. Stewart, L. M. Paradee, I. Raabe, N. Trapp, J. M. Slattery, I. Krossing, W. E. Geiger, J. Fluorine Chem. 2010, 131, 1091–1095.
- 15
- 15aI. Krossing, Chem. Eur. J. 2001, 7, 490–502;
10.1002/1521-3765(20010119)7:2<490::AID-CHEM490>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 15bThe experiment may be carried out by directly using NO[Al(ORF)4] as oxidant, which can be prepared by reaction of NOSbF6 with Li[Al(ORF)4] in liquid SO2. A. Decken, H. D. B. Jenkins, G. B. Nikiforov, J. Passmore, Dalton Trans. 2004, 2496–2504.
- 16Both the couplings of protons at CH2 and CH3 positions are related to the spin density of CH2 carbons, and their hyperfine coupling constants might be similar:
- 16aT. Shiga, J. Phys. Chem. 1965, 69, 3805–3814;
- 16bT. Shiga, A. Boukhors, P. Douzou, J. Phys. Chem. 1967, 71, 3559–3565.
- 17All calculations were performed using the Gaussian 09 program suite. The geometry optimizations were carried out at the (U)B3LYP/6-31G(d,p) level of theory. M. J. Frisch, et al. Gaussian 09, Revision B.01; Gaussian, Inc.:Wallingford, CT, 2010. See the Supporting Information for geometries and coordinates.
- 18X-ray data for [2-2]2+[Al(ORF)4]−2 (MoKα, λ=0.71073 Å): C34H18AlF36O6, FW=1233.46, orthorhombic, space group Pbca, Z=8, μ=0.244 mm−1, a=18.790(1), b=18.305(1), c=25.580(2) Å, T=123 (2) K, V=8797.8(13) Å3, R1=0.0494 for 5444 (I>2σ(I)) reflections, wR2=0.1295 (all data). CCDC 886759 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19P. R. Schreiner, L. V. Chernish, P. A. Gunchenko, E. Y. Tikhonchuk, H. Hausmann, M. Serafin, S. Schlecht, J. E. P. Dahl, R. M. K. Carlson, A. A. Fokin, Nature 2011, 477, 308–311.
- 20A. A. Fokin, L. V. Chernish, P. A. Gunchenko, E. Y. Tikhonchuk, H. Hausmann, M. Serafin, J. E. P. Dahl, R. M. K. Carlson, P. R. Schreiner, J. Am. Chem. Soc. 2012, 134, 13641–13650.
- 21F. Marchetti, G. Pampaloni, S. Zacchini, Dalton Trans. 2009, 8096–8106.
- 22Complexes [1-1]2+[Al(ORF)4]−2 and [3-3]2+[Al(ORF)4]−2 did not afford crystals suitable for X-ray crystallography, and thus no detailed structures were obtained.