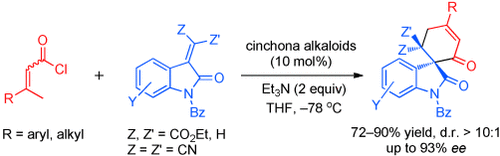
Catalytic [4+2] Cyclization of α,β-Unsaturated Acyl Chlorides with 3-Alkylenyloxindoles: Highly Diastereo- and Enantioselective Synthesis of Spirocarbocyclic Oxindoles†
Dr. Li-Tao Shen
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorWen-Qiang Jia
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Song Ye
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)Search for more papers by this authorDr. Li-Tao Shen
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorWen-Qiang Jia
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Song Ye
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)Search for more papers by this authorFinancial support from the Ministry of Science and Technology of China (2011CB808600), National Natural Science Foundation of China (No. 21072195), and the Chinese Academy of Sciences is gratefully acknowledged.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201207405_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Yang, X. Z. Wearing, P. W. Le Quesne, J. R. Deschamps, J. M. Cook, J. Nat. Prod. 2008, 71, 1431;
- 1bP. R. Sebahar, R. M. Williams, J. Am. Chem. Soc. 2000, 122, 5666;
- 1cT. Onishi, P. R. Sebahar, R. M. Williams, Org. Lett. 2003, 5, 3135;
- 1dS. Edmondson, S. J. Danishefsky, L. Sepp-Lorenzino, N. Rosen, J. Am. Chem. Soc. 1999, 121, 2147;
- 1eT. D. Bagul, G. Lakshmaiah, T. Kawabata, K. Fuji, Org. Lett. 2002, 4, 249;
- 1fK. A. Miller, S. Tsukamoto, R. M. Williams, Nat. Chem. 2009, 1, 63;
- 1gB. M. Trost, N. Cramer, H. Bernsmann, J. Am. Chem. Soc. 2007, 129, 3086;
- 1hK. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller, S. Wang, J. Med. Chem. 2006, 49, 3432.
- 2For reviews, see:
- 2aC. Marti, E. M. Carreira, Eur. J. Org. Chem. 2003, 2209;
- 2bC. V. Galliford, K. A. Scheidt, Angew. Chem. 2007, 119, 8902;
10.1002/ange.200701342 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8748;
- 2cB. M. Trost, M. K. Brennan, Synthesis 2009, 3003;
- 2dF. Zhou, Y.-L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381;
- 2eN. R. Ball-Jones, J. J. Badillo, A. K. Franz, Org. Biomol. Chem. 2012, 10, 5156;
- 2fR. Dalpozzo, G. Bencivenni, Chem. Soc. Rev. 2012, 41, 7247.
- 3
- 3aX. Z. Wearing, J. M. Cook, Org. Lett. 2002, 4, 4237;
- 3bR. M. Williams, J. Cao, H. Tsujishima, J. R. Cox, J. Am. Chem. Soc. 2003, 125, 12172;
- 3cG. D. Artman III, A. W. Grubbs, R. M. Williams, J. Am. Chem. Soc. 2007, 129, 6336;
- 3dP. S. Baran, T. J. Maimone, J. M. Richter, Nature 2007, 446, 404;
- 3eS. D. Edmondson, S. J. Danishefsky, Angew. Chem. 1998, 110, 1190;
10.1002/(SICI)1521-3757(19980420)110:8<1190::AID-ANGE1190>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1138;10.1002/(SICI)1521-3773(19980504)37:8<1138::AID-ANIE1138>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 3fH. Miyamoto, Y. Okawa, A. Nakazaki, S. Kobayashi, Angew. Chem. 2006, 118, 2332;
10.1002/ange.200504247 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2274;
- 3gM. Pettersson, D. Knueppel, S. F. Martin, Org. Lett. 2007, 9, 4623.
- 4
- 4aG. E. Keck, A. M. Tafesh, J. Org. Chem. 1989, 54, 5846;
- 4bT. Matsuura, L. E. Overman, D. J. Poon, J. Am. Chem. Soc. 1998, 120, 6500;
- 4cA. Huang, J. J. Kodanko, L. E. Overman, J. Am. Chem. Soc. 2004, 126, 14043;
- 4dL. E. Overman, M. D. Rosen, Angew. Chem. 2000, 112, 4768;
10.1002/1521-3757(20001215)112:24<4768::AID-ANGE4768>3.0.CO;2-7 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4596;10.1002/1521-3773(20001215)39:24<4596::AID-ANIE4596>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 4eH. Kamisaki, T. Nanjo, C. Tsukano, Y. Takemoto, Chem. Eur. J. 2011, 17, 626.
- 5
- 5aG. Palmisano, R. Annunziata, G. Pepeo, M. Sisti, Tetrahedron: Asymmetry 1996, 7, 1;
- 5bG. Cravotto, G. B. Giovenzana, T. Pilati, M. Sisti, G. Palmisno, J. Org. Chem. 2001, 66, 8447;
- 5cF. von Nussbaum, S. J. Danishefsky, Angew. Chem. 2000, 112, 2259;
10.1002/1521-3757(20000616)112:12<2259::AID-ANGE2259>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2175.10.1002/1521-3773(20000616)39:12<2175::AID-ANIE2175>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 6
- 6aB. M. Trost, N. Cramer, S. M. Silverman, J. Am. Chem. Soc. 2007, 129, 12396;
- 6bB. M. Trost, J. P. Stambuli, S. M. Silverman, U. Schwörer, J. Am. Chem. Soc. 2006, 128, 13328;
- 6cB. M. Trost, N. Cramer, H. Bernsmann, J. Am. Chem. Soc. 2007, 129, 3086.
- 7
- 7aG. R. Cook, P. S. Shanker, K. Pararajasingham, Angew. Chem. 1999, 111, 226;
10.1002/(SICI)1521-3757(19990115)111:1/2<226::AID-ANGE226>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 110;10.1002/(SICI)1521-3773(19990115)38:1/2<110::AID-ANIE110>3.0.CO;2-L CAS Web of Science® Google Scholar
- 7bC. Fischer, C. Meyers, E. M. Carreira, Helv. Chim. Acta 2000, 83, 1175;
- 7cA. Lerchner, E. M. Carreira, J. Am. Chem. Soc. 2002, 124, 14826;
- 7dC. Meyers, E. M. Carreira, Angew. Chem. 2003, 115, 718;
10.1002/ange.200390160 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 694.
- 8
- 8aG. Bencivenni, L.-Y. Wu, A. Mazzanti, B. Giannichi, F. Pesciaioli, M.-P. Song, G. Bartoli, P. Melchiorre, Angew. Chem. 2009, 121, 7336;
10.1002/ange.200903192 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7200;
- 8bY. Liu, M. Nappi, E. Arceo, S. Vera, P. Melchiorre, J. Am. Chem. Soc. 2011, 133, 15212.
- 9L.-L. Wang, L. Peng, J.-F. Bai, Q.-C. Huang, X.-Y. Xu, L.-X. Wang, Chem. Commun. 2010, 46, 8064.
- 10
- 10aK. Jiang, Z.-J. Jia, S. Chen, L. Wu, Y.-C. Chen, Chem. Eur. J. 2010, 16, 2852;
- 10bK. Jiang, Z.-J. Jia, X. Yin, L. Wu, Y.-C. Chen, Org. Lett. 2010, 12, 2766.
- 11F. Zhong, X. Han, Y. Wang, Y. Lu, Angew. Chem. 2011, 123, 7983; Angew. Chem. Int. Ed. 2011, 50, 7837.
- 12
- 12aP. S. Tiseni, R. Peters, Angew. Chem. 2007, 119, 5419;
10.1002/ange.200700859 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5325;
- 12bP. S. Tiseni, R. Peters, Org. Lett. 2008, 10, 2019;
- 12cP. S. Tiseni, R. Peters, Chem. Eur. J. 2010, 16, 2503.
- 13
- 13aL.-T. Shen, P.-L. Shao, S. Ye, Adv. Synth. Catal. 2011, 353, 1943;
- 13bT. Wang, S. Ye, Sci. Sin. Chim. 2011, 41, 13061.
- 14For reviews on NHC catalysis, see:
- 14aD. Enders, T. Balensiefer, Acc. Chem. Res. 2004, 37, 534;
- 14bD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606;
- 14cV. Nair, S. Vellalath, B. P. Babu, Chem. Soc. Rev. 2008, 37, 2691;
- 14dJ. L. Moore, T. Rovis, Top. Curr. Chem. 2010, 291, 77;
- 14eV. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, Chem. Soc. Rev. 2011, 40, 5336;
- 14fA. Grossmann, D. Enders, Angew. Chem. 2012, 124, 320; Angew. Chem. Int. Ed. 2012, 51, 314.
- 15L.-T. Shen, L.-H. Sun, S. Ye, J. Am. Chem. Soc. 2011, 133, 15894.
- 16The crystal was prepared from the solution in petroleum ether/ethyl acetate, with a trace of chloroform. CCDC 900492 ((+)-3 a), 892111 (rac-3 i) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17The epimerization of trans-3 to cis-3 under the reaction conditions was observed with elongated reaction time, which accounts for the presence of some cis-product in some cases.