Asymmetric Ion-Pairing Catalysis
Dr. Katrien Brak
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St, Cambridge MA 02138 (USA)
Search for more papers by this authorCorresponding Author
Prof. Eric N. Jacobsen
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St, Cambridge MA 02138 (USA)
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St, Cambridge MA 02138 (USA)Search for more papers by this authorDr. Katrien Brak
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St, Cambridge MA 02138 (USA)
Search for more papers by this authorCorresponding Author
Prof. Eric N. Jacobsen
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St, Cambridge MA 02138 (USA)
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford St, Cambridge MA 02138 (USA)Search for more papers by this authorGraphical Abstract
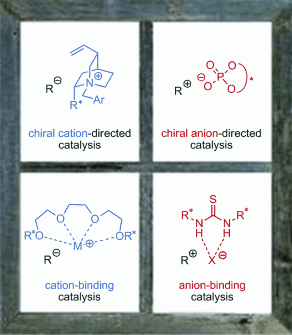
Framing the field, this review provides an overview of four ion-pairing strategies that have emerged for asymmetric catalysis of transformations proceeding through charged intermediates or reagents (see picture). Particular emphasis is placed on the mechanistic features that enable high asymmetric induction in these systems.
Abstract
Charged intermediates and reagents are ubiquitous in organic transformations. The interaction of these ionic species with chiral neutral, anionic, or cationic small molecules has emerged as a powerful strategy for catalytic, enantioselective synthesis. This review describes developments in the burgeoning field of asymmetric ion-pairing catalysis with an emphasis on the insights that have been gleaned into the structural and mechanistic features that contribute to high asymmetric induction.
References
- 1
- 1aN. Bjerrum, K. Dan. Videsk. Selesk. Math.-Fys. Medd. 1926, 7, 3;
- 1bM. Szwarc, Acc. Chem. Res. 1969, 2, 87–96.
- 2E. V. Anslyn, D. A. Dougherty, Modern Physical Organic Chemistry, University Science Books, Sausalito, 2006.
- 3
- 3aS. Winstein, E. Clippinger, A. H. Fainberg, G. C. Robinson, J. Am. Chem. Soc. 1954, 76, 2597–2598;
- 3bH. Sadek, R. M. Fuoss, J. Am. Chem. Soc. 1954, 76, 5897–5901.
- 4
- 4aM. S. Taylor, E. N. Jacobsen, Angew. Chem. 2006, 118, 1550–1573; Angew. Chem. Int. Ed. 2006, 45, 1520–1543;
- 4bA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713–5743;
- 4cM. Terada, Synthesis 2010, 1929–1982;
- 4dT. Akiyama, Chem. Rev. 2007, 107, 5744–5758.
- 5
- 5aM. J. O’Donnell, Acc. Chem. Res. 2004, 37, 506–517;
- 5bB. Lygo, B. I. Andrews, Acc. Chem. Res. 2004, 37, 518–525;
- 5cT. Hashimoto, K. Maruoka, Chem. Rev. 2007, 107, 5656–5682;
- 5dT. Ooi, K. Maruoka, Angew. Chem. 2007, 119, 4300–4345;
10.1002/ange.200601737 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4222–4266;
- 5eS.-s. Jew, H.-g. Park, Chem. Commun. 2009, 7090–7103;
- 5fK. Maruoka, Asymmetric Phase Transfer Catalysis, Wiley-VCH, New York, 2008;
10.1002/9783527622627 Google Scholar
- 5gI. Ojima, Catalytic Asymmetric Synthesis, 3rd ed., Wiley-VCH, New York, 2010;
10.1002/9780470584248 Google Scholar
- 5hT. Ooi, K. Maruoka, Acc. Chem. Res. 2004, 37, 526–533;
- 5iU.-H. Dolling, P. Davis, E. J. J. Grabowski, J. Am. Chem. Soc. 1984, 106, 446–447.
- 6
- 6aJ. Lacour, D. Moraleda, Chem. Commun. 2009, 7073–7089;
- 6bG. Adair, S. Mukherjee, B. List, Aldrichimica Acta 2008, 41, 31–39.
- 7Z. Zhang, P. R. Schreiner, Chem. Soc. Rev. 2009, 38, 1187–1198.
- 8For a recent review on chiral anions in asymmetric catalysis, see: R. J. Phipps, G. L. Hamilton, F. D. Toste, Nat. Chem. 2012, 4, 603–614.
- 9M. J. O’Donnell, W. D. Bennett, S. Wu, J. Am. Chem. Soc. 1989, 111, 2353–2355.
- 10M. Rabinovitz, Y. Cohen, M. Halpern, Angew. Chem. 1986, 98, 958–968; Angew. Chem. Int. Ed. Engl. 1986, 25, 960–970.
- 11D. L. Hughes, U.-H. Dolling, K. M. Ryan, E. F. Schoenwaldt, E. J. J. Grabowski, J. Org. Chem. 1987, 52, 4745–4752.
- 12S. E. Denmark, R. C. Weintraub, Heterocycles 2010, 82, 1527–1540.
- 13E. J. Corey, F. Xu, M. C. Noe, J. Am. Chem. Soc. 1997, 119, 12414–12415.
- 14B. Lygo, P. G. Wainwright, Tetrahedron Lett. 1997, 38, 8595–8598.
- 15C. Hofstetter, P. S. Wilkinson, T. C. Pochapsky, J. Org. Chem. 1999, 64, 8794–8800.
- 16M. T. Reetz, Angew. Chem. 1988, 100, 1026–1030; Angew. Chem. Int. Ed. Engl. 1988, 27, 994–998.
- 17
- 17aM. T. Reetz, S. Hutte, R. Goddard, J. Am. Chem. Soc. 1993, 115, 9339–9340;
- 17bM. T. Reetz, S. Hutte, R. Goddard, C. Robyr, Chem. Eur. J. 1996, 2, 382–384.
- 18C. E. Cannizzaro, K. N. Houk, J. Am. Chem. Soc. 2002, 124, 7163–7169.
- 19K. B. Lipkowitz, M. W. Cavanaugh, B. Baker, M. J. O’Donnell, J. Org. Chem. 1991, 56, 5181–5192.
- 20For an alternative, qualitative model that invokes a perpendicular association of the enolate to the tetrahedron face, see ref. [13].
- 21S.-s. Jew, M.-S. Yoo, B.-S. Jeong, Y. I. Park, H.-g. Park, Org. Lett. 2002, 4, 4245–4248.
- 22M.-S. Yoo, B.-S. Jeong, J.-H. Lee, H.-g. Park, S.-s. Jew, Org. Lett. 2005, 7, 1129–1131.
- 23V. R. Thalladi, H. Weiss, D. Blaeser, R. Boese, A. Nangia, G. R. Desiraju, J. Am. Chem. Soc. 1998, 120, 8702–8710.
- 24K. Maruoka, T. Ooi, T. Kano, Chem. Commun. 2007, 1487–1495.
- 25
- 25aT. Ohshima, T. Shibuguchi, Y. Fukuta, M. Shibasaki, Tetrahedron 2004, 60, 7743–7754;
- 25bA. Okada, T. Shibuguchi, T. Ohshima, H. Masu, K. Yamaguchi, M. Shibasaki, Angew. Chem. 2005, 117, 4640–4643; Angew. Chem. Int. Ed. 2005, 44, 4564–4567.
- 26Other binaphthyl-based chiral quaternary ammonium catalysts have also been developed, see:
- 26aT. Ooi, Y. Uematsu, M. Kameda, K. Maruoka, Angew. Chem. 2002, 114, 1621–1624;
10.1002/1521-3757(20020503)114:9<1621::AID-ANGE1621>3.0.CO;2-5 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1551–1554;10.1002/1521-3773(20020503)41:9<1551::AID-ANIE1551>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 26bM. Kitamura, S. Shirakawa, K. Maruoka, Angew. Chem. 2005, 117, 1573–1575;
10.1002/ange.200462257 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1549–1551.
- 27
- 27aT. Shibuguchi, Y. Fukuta, Y. Akachi, A. Sekine, T. Ohshima, M. Shibasaki, Tetrahedron Lett. 2002, 43, 9539–9543;
- 27bT. Ooi, M. Kameda, K. Maruoka, J. Am. Chem. Soc. 1999, 121, 6519–6520.
- 28T. Ooi, M. Kameda, K. Maruoka, J. Am. Chem. Soc. 2003, 125, 5139–5151.
- 29J. Halpern, Science 1982, 217, 401–407.
- 30R. He, X. Wang, T. Hashimoto, K. Maruoka, Angew. Chem. 2008, 120, 9608–9610; Angew. Chem. Int. Ed. 2008, 47, 9466–9468.
- 31R. He, C. Ding, K. Maruoka, Angew. Chem. 2009, 121, 4629–4631; Angew. Chem. Int. Ed. 2009, 48, 4559–4561.
- 32D. Uraguchi, Y. Asai, T. Ooi, Angew. Chem. 2009, 121, 747–751; Angew. Chem. Int. Ed. 2009, 48, 733–737.
- 33
- 33aD. Uraguchi, T. Ito, T. Ooi, J. Am. Chem. Soc. 2009, 131, 3836–3837;
- 33bD. Uraguchi, S. Sakaki, T. Ooi, J. Am. Chem. Soc. 2007, 129, 12392–12393;
- 33cD. Uraguchi, Y. Ueki, T. Ooi, J. Am. Chem. Soc. 2008, 130, 14088–14089;
- 33dD. Uraguchi, Y. Ueki, T. Ooi, Science 2009, 326, 120–123.
- 34For examples of other cationic phase-transfer catalysts that interact with anions primarily via H-bonding, see:
- 34aT. Kita, A. Georgieva, Y. Hashimoto, T. Nakata, K. Nagasawa, Angew. Chem. 2002, 114, 2956–2958;
Angew. Chem. Int. Ed. 2002, 41, 2832–2834;
10.1002/1521-3773(20020802)41:15<2832::AID-ANIE2832>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 34bK. Ohmatsu, M. Kiyokawa, T. Ooi, J. Am. Chem. Soc. 2011, 133, 1307–1309.
- 35For a comprehensive review of PTC by polyether catalysts, as well as other metal cation complexing agents such as TADDOL, NOBIN, and metal(salen)complexes, see ref. [5d,f].
- 36D. J. Cram, G. D. Y. Sogah, J. Chem. Soc. Chem. Commun. 1981, 625–628.
- 37T. Akiyama, M. Hara, K. Fuchibe, S. Sakamoto, K. Yamaguchi, Chem. Commun. 2003, 1734–1735.
- 38H. Yan, H. B. Jang, J.-W. Lee, H. K. Kim, S. W. Lee, J. W. Yang, C. E. Song, Angew. Chem. 2010, 122, 9099–9101; Angew. Chem. Int. Ed. 2010, 49, 8915–8917.
- 39W. J. Lee, H. Yan, H. B. Jang, H. K. Kim, S.-W. Park, S. Lee, D. Y. Chi, C. E. Song, Angew. Chem. 2009, 121, 7819–7822; Angew. Chem. Int. Ed. 2009, 48, 7683–7686.
- 40M. Fleischmann, D. Drettwan, E. Sugiono, M. Rueping, R. M. Gschwind, Angew. Chem. 2011, 123, 6488–6493;
10.1002/ange.201101385 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6364–6369.
- 41D. B. Llewellyn, D. Adamson, B. A. Arndtsen, Org. Lett. 2000, 2, 4165–4168.
- 42D. B. Llewellyn, B. A. Arndtsen, Tetrahedron: Asymmetry 2005, 16, 1789–1799.
- 43C. Carter, S. Fletcher, A. Nelson, Tetrahedron: Asymmetry 2003, 14, 1995–2004.
- 44G. L. Hamilton, T. Kanai, F. D. Toste, J. Am. Chem. Soc. 2008, 130, 14984–14986.
- 45T. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. 2004, 116, 1592–1594;
10.1002/ange.200353240 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1566–1568.
- 46D. Uraguchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 5356–5357.
- 47S. Mayer, B. List, Angew. Chem. 2006, 118, 4299–4301; Angew. Chem. Int. Ed. 2006, 45, 4193–4195.
- 48
- 48aJ. W. Yang, M. T. H. Fonseca, N. Vignola, B. List, Angew. Chem. 2005, 117, 110–112; Angew. Chem. Int. Ed. 2005, 44, 108–110;
- 48bS. G. Ouellet, J. B. Tuttle, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 32–33.
- 49M. Stadler, B. List, Synlett 2008, 597–599.
- 50L. Simón, J. M. Goodman, J. Am. Chem. Soc. 2008, 130, 8741–8747.
- 51X. Wang, B. List, Angew. Chem. 2008, 120, 1135–1138; Angew. Chem. Int. Ed. 2008, 47, 1119–1122.
- 52
- 52aM. Marigo, J. Franzen, T. B. Poulsen, W. Zhuang, K. A. Jorgensen, J. Am. Chem. Soc. 2005, 127, 6964–6965;
- 52bC. Sparr, W. B. Schweizer, H. M. Senn, R. Gilmour, Angew. Chem. 2009, 121, 3111–3114;
10.1002/ange.200900405 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3065–3068.
- 53
- 53aN. J. A. Martin, B. List, J. Am. Chem. Soc. 2006, 128, 13368–13369;
- 53bJ. Zhou, B. List, J. Am. Chem. Soc. 2007, 129, 7498–7499;
- 53cX. Wang, C. M. Reisinger, B. List, J. Am. Chem. Soc. 2008, 130, 6070–6071.
- 54O. Lifchits, C. M. Reisinger, B. List, J. Am. Chem. Soc. 2010, 132, 10227–10229.
- 55B. E. Maryanoff, H.-C. Zhang, J. H. Cohen, I. J. Turchi, C. A. Maryanoff, Chem. Rev. 2004, 104, 1431–1628.
- 56M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt, D. J. Dixon, J. Am. Chem. Soc. 2009, 131, 10796–10797.
- 57C. A. Holloway, M. E. Muratore, R. I. Storer, D. J. Dixon, Org. Lett. 2010, 12, 4720–4723.
- 58Y. Xie, Y. Zhao, B. Qian, L. Yang, C. Xia, H. Huang, Angew. Chem. 2011, 123, 5800–5804; Angew. Chem. Int. Ed. 2011, 50, 5682–5686.
- 59M. J. Wanner, R. N. S. van der Haas, K. R. de Cuba, J. H. van Maarseveen, H. Hiemstra, Angew. Chem. 2007, 119, 7629–7631;
10.1002/ange.200701808 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7485–7487.
- 60N. V. Sewgobind, M. J. Wanner, S. Ingemann, R. de Gelder, J. H. van Maarseveen, H. Hiemstra, J. Org. Chem. 2008, 73, 6405–6408.
- 61M. J. Wanner, R. N. A. Boots, B. Eradus, R. de Gelder, J. H. van Maarseveen, H. Hiemstra, Org. Lett. 2009, 11, 2579–2581.
- 62Indolyl N-tosyl amines have also been used as carbocation precursors: F.-L. Sun, X.-J. Zheng, Q. Gu, Q.-L. He, S.-L. You, Eur. J. Org. Chem. 2010, 47–50.
- 63F.-L. Sun, M. Zeng, Q. Gu, S.-L. You, Chem. Eur. J. 2009, 15, 8709–8712.
- 64Q.-X. Guo, Y.-G. Peng, J.-W. Zhang, L. Song, Z. Feng, L.-Z. Gong, Org. Lett. 2009, 11, 4620–4623.
- 65T. Liang, Z. Zhang, J. C. Antilla, Angew. Chem. 2010, 122, 9928–9930; Angew. Chem. Int. Ed. 2010, 49, 9734–9736.
- 66G. Bergonzini, S. Vera, P. Melchiorre, Angew. Chem. 2010, 122, 9879–9882;
10.1002/ange.201004761 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9685–9688.
- 67I. Čorić, S. Vellalath, B. List, J. Am. Chem. Soc. 2010, 132, 8536–8637.
- 68I. Čorić, S. Muller, B. List, J. Am. Chem. Soc. 2010, 132, 17370–17373.
- 69M. Terada, H. Tanaka, K. Sorimachi, J. Am. Chem. Soc. 2009, 131, 3430–3431.
- 70Q.-W. Zhang, C.-A. Fan, H.-J. Zhang, Y.-Q. Tu, Y.-M. Zhao, P. Gu, Z.-M. Chen, Angew. Chem. 2009, 121, 8724–8726; Angew. Chem. Int. Ed. 2009, 48, 8572–8574.
- 71
- 71aY. Takeuchi, E. Suzuki, N. Shibata, J. Am. Chem. Soc. 2000, 122, 10728–10729;
- 71bD. Cahard, C. Audouard, J.-C. Plaquevent, N. Roques, Org. Lett. 2000, 2, 3699–3701;
- 71cT. Ishimaru, N. Shibata, T. Horikawa, N. Yasuda, S. Nakamura, T. Toru, M. Shiro, Angew. Chem. 2008, 120, 4225–4229;
10.1002/ange.200800717 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4157–4161;
- 71dO. Lozano, G. Blessley, T. M. del Campo, A. L. Thompson, G. T. Giuffredi, M. Bettati, M. Walker, R. Borman, V. Gouverneur, Angew. Chem. 2011, 123, 8255–8259; Angew. Chem. Int. Ed. 2011, 50, 8105–8109.
- 72V. Rauniyar, A. D. Lackner, G. L. Hamilton, F. D. Toste, Science 2011, 334, 1681–1684.
- 73
- 73aR. J. Phipps, K. Hiramatsu, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 8376–8379;
- 73bA phosphoric acid-catalyzed bromination of enecarbamates that may proceed via asymmetric ion-pairing catalysis was also reported: A. Alix, C. Lalli, P. Retailleau, G. Masson, J. Am. Chem. Soc. 2012, 134, 10389–10392.
- 74U. Hennecke, C. H. Muller, R. Frolich, Org. Lett. 2011, 13, 860–863.
- 75Additional reports of chiral phosphoric acid-catalyzed bromocycloetherifications that may proceed via ion-pairing catalysis:
- 75aD. Huang, H. Wang, F. Xue, H. Guan, L. Li, X. Peng, Y. Shi, Org. Lett. 2011, 13, 6350–6353;
- 75bS. E. Denmark, M. T. Burk, Org. Lett. 2012, 14, 256–259.
- 76G. L. Hamilton, E. J. Kang, M. Mba, F. D. Toste, Science 2007, 317, 496–499.
- 77R. L. LaLonde, Z. J. Wang, M. Mba, A. D. Lackner, F. D. Toste, Angew. Chem. 2010, 122, 608–611;
10.1002/ange.200905000 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 598–601.
- 78For a review on the effects of achiral counterions on chiral ionic transition-metal complexes, see: A. Macchioni, Chem. Rev. 2005, 105, 2039–2073.
- 79Examples of transition-metal catalysis with chiral phosphates:
- 79aH. Alper, N. Hamel, J. Am. Chem. Soc. 1990, 112, 2803–2804;
- 79bS. Mukherjee, B. List, J. Am. Chem. Soc. 2007, 129, 11336–11337;
- 79cB. Zhao, H. Du, Y. Shi, J. Org. Chem. 2009, 74, 8392–8395;
- 79dR. Yazaki, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 10275–10277;
- 79eS. Liao, B. List, Angew. Chem. 2010, 122, 638–641; Angew. Chem. Int. Ed. 2010, 49, 628–631;
- 79fV. Rauniyar, Z. J. Wang, H. E. Burks, F. D. Toste, J. Am. Chem. Soc. 2011, 133, 8486–8489;
- 79gG. Jiang, R. Halder, Y. Fang, B. List, Angew. Chem. 2011, 123, 9926–9929; Angew. Chem. Int. Ed. 2011, 50, 9752–9755;
- 79hG. Jiang, B. List, Angew. Chem. 2011, 123, 9643–9646; Angew. Chem. Int. Ed. 2011, 50, 9471–9474;
- 79iG. Jiang, B. List, Chem. Commun. 2011, 47, 10022–10024;
- 79jJ. R. Zbieg, E. Yamaguchi, E. L. McInturff, M. J. Krische, Science 2012, 336, 324–327.
- 80Examples of cooperative transition metal and Bronsted-acid catalysis:
- 80aV. Komanduri, M. J. Krische, J. Am. Chem. Soc. 2006, 128, 16448–16449;
- 80bM. Rueping, A. P. Antonchick, C. Brinkmann, Angew. Chem. 2007, 119, 7027–7030;
10.1002/ange.200702439 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6903–6906;
- 80cW. Hu, X. Xu, J. Zhou, W.-J. Liu, H. Huang, J. Hu, L. Yang, L.-Z. Gong, J. Am. Chem. Soc. 2008, 130, 7782–7783;
- 80dC. Li, C. Wang, B. Villa-Marcos, J. Xiao, J. Am. Chem. Soc. 2008, 130, 14450–14451;
- 80eC. Li, B. Villa-Marcos, J. Xiao, J. Am. Chem. Soc. 2009, 131, 6967–6969.
- 81Z. Yu, W. Jin, Q. Jiang, Angew. Chem. Int. Ed. 2012, 51, 6060–6072.
- 82M. Rueping, R. M. Koenigs, I. Atodiresei, Chem. Eur. J. 2010, 16, 9350–9365.
- 83J. Meeuwissen, J. N. H. Reek, Nat. Chem. 2010, 2, 615–621.
- 84K. Ohmatsu, M. Ito, T. Kunieda, T. Ooi, Nat. Chem. 2012, 4, 473–477.
- 85D. Nakashima, H. Yamamoto, J. Am. Chem. Soc. 2006, 128, 9626–9627.
- 86M. Rueping, B. J. Nachtsheim, W. Ieawsuwan, I. Atodiresei, Angew. Chem. 2011, 123, 6838–6853;
10.1002/ange.201100169 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6706–6720.
- 87P. Christ, A. G. Lindsay, S. S. Vormittag, J.-M. Neudorfl, A. Berkessel, A. C. O’Donoghue, Chem. Eur. J. 2011, 17, 8524–8528.
- 88M. Rueping, B. J. Nachtsheim, S. A. Moreth, M. Bolte, Angew. Chem. 2008, 120, 603–606;
10.1002/ange.200703668 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 593–596.
- 89M. Rueping, B. J. Nachtsheim, Synlett 2010, 119–122.
- 90M. Rueping, U. Uria, M.-Y. Lin, I. Atodiresei, J. Am. Chem. Soc. 2011, 133, 3732–3735.
- 91C. H. Cheon, H. Yamamoto, J. Am. Chem. Soc. 2008, 130, 9246–9247.
- 92Another example of asymmetric ion-pairing catalysis if desilylation is rate and ee-determining: M. Sickert, C. Schneider, Angew. Chem. 2008, 120, 3687–3690;
10.1002/ange.200800103 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3631–3634.
- 93R. I. Storer, D. E. Carrera, Y. Ni, D. W. C. MacMillan, J. Am. Chem. Soc. 2006, 128, 84–86.
- 94M. Sickert, F. Abels, M. Lang, J. Sieler, C. Birkemeyer, C. Schneider, Chem. Eur. J. 2010, 16, 2806–2818.
- 95M. Terada, Chem. Commun. 2008, 4097–4112.
- 96
- 96aI. D. Gridnev, M. Kouchi, K. Sorimachi, M. Terada, Tetrahedron Lett. 2007, 48, 497–500;
- 96bL. Simón, J. M. Goodman, J. Org. Chem. 2011, 76, 1775–1788.
- 97M. Yamanaka, J. Itoh, K. Fuchibe, T. Akiyama, J. Am. Chem. Soc. 2007, 129, 6756–6764.
- 98
- 98aP. García-García, F. Lay, P. Garcia-Garcia, C. Rabalakos, B. List, Angew. Chem. 2009, 121, 4427–4430;
10.1002/ange.200901768 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4363–4366;
- 98bL. Ratjen, P. Garcia-Garcia, F. Lay, M. E. Beck, B. List, Angew. Chem. 2011, 123, 780–784; Angew. Chem. Int. Ed. 2011, 50, 754–758.
- 99I. Čorić, B. List, Nature 2012, 483, 315–319.
- 100S. Liao, E. J. Corey, W. Qinggang, B. List, J. Am. Chem. Soc. 2012, 134, 10765–10768.
- 101
- 101aF. P. Schmidtchen, M. Berger, Chem. Rev. 1997, 97, 1609–1646;
- 101bG. W. Bates, M. E. Light, M. Albrecht, P. A. Gale, J. Org. Chem. 2007, 72, 8921–8927.
- 102
- 102aA. A. Rodriguez, H. Yoo, J. W. Ziller, K. J. Shea, Tetrahedron Lett. 2009, 50, 6830–6833;
- 102bB. P. Hay, Chem. Soc. Rev. 2010, 39, 3700–3708.
- 103M. Kotke, P. R. Schreiner, Tetrahedron 2006, 62, 434–439.
- 104D. J. Tantillo, K. N. Houk, J. Comput. Chem. 2002, 23, 84–95.
- 105M. Kotke, P. R. Schreiner, Synthesis 2007, 779–790.
- 106
- 106aM. S. Sigman, E. N. Jacobsen, J. Am. Chem. Soc. 1998, 120, 4901–4902;
- 106bM. S. Sigman, P. Vachal, E. N. Jacobsen, Angew. Chem. 2000, 112, 1336–1338;
Angew. Chem. Int. Ed. 2000, 39, 1279–1281;
10.1002/(SICI)1521-3773(20000403)39:7<1279::AID-ANIE1279>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 106cP. Vachal, E. N. Jacobsen, Org. Lett. 2000, 2, 867–870.
- 107S. J. Zuend, M. P. Coughlin, M. Lalonde, E. N. Jacobsen, Nature 2009, 461, 968–971.
- 108S. J. Zuend, E. N. Jacobsen, J. Am. Chem. Soc. 2009, 131, 15358–15374.
- 109C. S. Pan, B. List, Chem. Asian J. 2008, 3, 430–437.
- 110
- 110aD. A. Jose, A. Singh, A. Das, B. Ganguly, Tetrahedron Lett. 2007, 48, 3695–3698;
- 110bS. Nishizawa, P. Buhlmann, M. Iwao, Y. Umezawa, Tetrahedron Lett. 1995, 36, 6483–6486;
- 110cA. Allerhand, P. v. R. Schleyer, J. Am. Chem. Soc. 1963, 85, 1233–1237.
- 111J. Stöckigt, A. P. Antonchick, F. Wu, H. Waldmann, Angew. Chem. 2011, 123, 8692–8719;
10.1002/ange.201008071 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8538–8564.
- 112M. S. Taylor, E. N. Jacobsen, J. Am. Chem. Soc. 2004, 126, 10558–10559.
- 113D. J. Mergott, S. J. Zuend, E. N. Jacobsen, Org. Lett. 2008, 10, 745–748.
- 114M. S. Taylor, N. Tokunaga, E. N. Jacobsen, Angew. Chem. 2005, 117, 6858–6862; Angew. Chem. Int. Ed. 2005, 44, 6700–6704.
- 115I. T. Raheem, P. S. Thiara, E. A. Peterson, E. N. Jacobsen, J. Am. Chem. Soc. 2007, 129, 13404–13405.
- 116I. T. Raheem, P. S. Thiara, E. N. Jacobsen, Org. Lett. 2008, 10, 1577–1580.
- 117E. A. Peterson, E. N. Jacobsen, Angew. Chem. 2009, 121, 6446–6449;
10.1002/ange.200902420 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6328–6331.
- 118Y. Yamaoka, H. Miyabe, Y. Takemoto, J. Am. Chem. Soc. 2007, 129, 6686–6687.
- 119R. R. Knowles, S. Lin, E. N. Jacobsen, J. Am. Chem. Soc. 2010, 132, 5030–5032.
- 120R. Thoma, T. Schulz-Gasch, B. D’Arcy, J. Benz, J. Aebi, H. Dehmlow, M. Hennig, M. Stihle, A. Ruf, Nature 2004, 432, 118–122.
- 121C. T. Calderone, D. H. Williams, J. Am. Chem. Soc. 2001, 123, 6262–6267.
- 122S. E. Reisman, A. G. Doyle, E. N. Jacobsen, J. Am. Chem. Soc. 2008, 130, 7198–7199.
- 123A. R. Brown, W.-H. Kuo, E. N. Jacobsen, J. Am. Chem. Soc. 2010, 132, 9286–9288.
- 124J. A. Birrell, J.-N. Desrosiers, E. N. Jacobsen, J. Am. Chem. Soc. 2011, 133, 13872–13875.
- 125T. R. Kelly, M. H. Kim, J. Am. Chem. Soc. 1994, 116, 7072–7080.
- 126A nonstereoselective application of this principle: T. Weil, M. Kotke, C. M. Kleiner, P. R. Schreiner, Org. Lett. 2008, 10, 1513–1516.
- 127R. S. Klausen, E. N. Jacobsen, Org. Lett. 2009, 11, 887–890.
- 128Y. Lee, R. S. Klausen, E. N. Jacobsen, Org. Lett. 2011, 13, 5564–5567.
- 129R. S. Klausen, A. M. Hyde, E. N. Jacobsen, unpublished results.
- 130J. Seayad, A. M. Seayad, B. List, J. Am. Chem. Soc. 2006, 128, 1086–1087.
- 131N. Z. Burns, M. R. Witten, E. N. Jacobsen, J. Am. Chem. Soc. 2011, 133, 14578–14581.
- 132G. C. Fu, Acc. Chem. Res. 2004, 37, 542–547.
- 133
- 133aK. C. De, E. G. Klauber, D. Seidel, J. Am. Chem. Soc. 2009, 131, 17060–17061;
- 133bE. G. Klauber, K. C. De, T. K. Shah, D. Seidel, J. Am. Chem. Soc. 2010, 132, 13624–13626;
- 133cN. Mittal, D. X. Sun, D. Seidel, Org. Lett. 2012, 14, 3084–3087.
- 134E. G. Klauber, N. Mittal, T. K. Shah, D. Seidel, Org. Lett. 2011, 13, 2464–2467.
- 135K. C. De, D. Seidel, J. Am. Chem. Soc. 2011, 133, 14538–14541.
- 136K. C. De, N. Mittal, D. Seidel, J. Am. Chem. Soc. 2011, 133, 16802–16805.
- 137G. E. Veitch, E. N. Jacobsen, Angew. Chem. 2010, 122, 7490–7493;
10.1002/ange.201003681 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7332–7335.
- 138H. Xu, S. J. Zuend, M. G. Woll, Y. Tao, E. N. Jacobsen, Science 2010, 327, 986–990.
- 139R. R. Knowles, E. N. Jacobsen, Proc. Natl. Acad. Sci. USA 2010, 107, 20678–20685.
- 140
- 140aL. Pauling, Chem. Eng. News 1946, 24, 1377;
- 140bW. P. Jencks, Catalysis in Chemistry and Enzymology, McGraw Hill, New York, 1989.
- 141M. G. Chudzinski, C. A. McClary, M. S. Taylor, J. Am. Chem. Soc. 2011, 133, 10559–10567.
- 142K. N. Houk, P. H.-Y. Cheong, Nature 2008, 455, 309–313.