Homochiral Crystallization of Metal–Organic Silver Frameworks: Asymmetric [3+2] Cycloaddition of an Azomethine Ylide†
Xu Jing
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorDr. Cheng He
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorDapeng Dong
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorLinlin Yang
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Chunying Duan
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)Search for more papers by this authorXu Jing
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorDr. Cheng He
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorDapeng Dong
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorLinlin Yang
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Chunying Duan
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116023 (P.R. China)Search for more papers by this authorWe gratefully acknowledge financial support from the NSFC (21171029 and 21025102).
Graphical Abstract
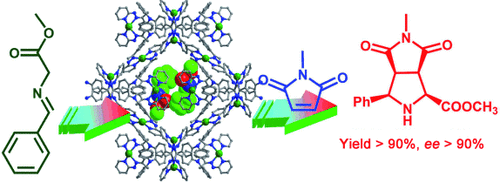
Enantiomeric silver-based MOFs were obtained through a homochiral crystallization of cinchonine and cinchonidine enantiomers as chiral adducts with silver. These MOFs exhibited excellent catalytic activity for asymmetric [3+2] cycloaddition (see scheme), giving products with high enantioselectivity.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201204530_sm_miscellaneous_information.pdf750.8 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aO. K. Farha, J. T. Hupp, Acc. Chem. Res. 2010, 43, 1166–1175;
- 1bM. D. Allendorf, C. A. Bauer, R. K. Bhakta, R. J. T. Houk, Chem. Soc. Rev. 2009, 38, 1330–1352;
- 1cY.-B. Zhang, W. X. Zhang, F. Y. Feng, J. P. Zhang, X. M. Chen, Angew. Chem. 2009, 121, 5391; Angew. Chem. Int. Ed. 2009, 48, 5287–5290;
- 1dM. Kurmoo, Chem. Soc. Rev. 2009, 38, 1353–1379;
- 1eJ. R. Li, R. J. Kuppler, H. C. Zhou, Chem. Soc. Rev. 2009, 38, 1477–1504;
- 1fD. Maspoch, D. RuizMolina, J. Veciana, Chem. Soc. Rev. 2007, 36, 770–818.
- 2
- 2aA. Corma, H. García, F. X. L. Xamena, Chem. Rev. 2010, 110, 4606–4655;
- 2bJ. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen, J. T. Hupp, Chem. Soc. Rev. 2009, 38, 1450–1459;
- 2cD. Farrusseng, S. Aguado, C. Pinel, Angew. Chem. 2009, 121, 7638–7649;
10.1002/ange.200806063 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7502–7513;
- 2dA. U. Czaja, N. Trukhan, U. Müller, Chem. Soc. Rev. 2009, 38, 1284–1293.
- 3
- 3aL. Yang, S. Kinoshita, T. Yamada, S. Kanda, H. Kitagawa, M. Tokunaga, T. Ishimoto, T. Ogura, R. Nagumo, A. Miyamoto, M. Koyama, Angew. Chem. 2010, 122, 5476–5479; Angew. Chem. Int. Ed. 2010, 49, 5348–5351;
- 3bE. Quartapelle Procopio, F. Linares, C. Montoro, V. Colombo, A. Maspero, E. Barea, J. A. R. Navarro, Angew. Chem. 2010, 122, 7466–7469;
10.1002/ange.201003314 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7308–7311;
- 3cC. Chizallet, S. Lazare, D. Bazer-Bachi, F. Bonnier, V. Lecocq, E. Soyer, A. A. Quoineaud, N. Bats, J. Am. Chem. Soc. 2010, 132, 12365–12377;
- 3dC. Wang, Z. G. Xie, K. E. deKrafft, W. B. Lin, J. Am. Chem. Soc. 2011, 133, 13445–13454.
- 4
- 4aM. Yoon, R. Srirambalaji, K. Kim, Chem. Rev. 2012, 112, 1196–1231;
- 4bG. Nickerl, A. Henschel, R. Grünker, K. Gedrich, S. Kaskel, Chem. Ing. Tech. 2011, 83, 90–103;
- 4cY. Liu, W. M. Xuan, Y. Cui, Adv. Mater. 2010, 22, 4112–4135;
- 4dL. Ma, C. Abney, W. B. Lin, Chem. Soc. Rev. 2009, 38, 1248–1256;
- 4eM. Heitbaum, F. Glorius, I. Escher, Angew. Chem. 2006, 118, 4850–4881;
10.1002/ange.200504212 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4732–4762.
- 5
- 5aL. Ma, J. M. Falkowski, C. Abney, W. B. Lin, Nat. Chem. 2010, 2, 838–846;
- 5bD. B. Dang, P. Y. Wu, C. He, Z. Xie, C. Y. Duan, J. Am. Chem. Soc. 2010, 133, 14566–14568;
- 5cT. Kawasaki, S. Kamimura, A. Amihara, K. Suzuki, K. Soai, Angew. Chem. 2011, 123, 6928–6930; Angew. Chem. Int. Ed. 2011, 50, 6796–6798.
- 6
- 6aD. J. Lun, G. I. N. Waterhouse, S. G. Telfer, J. Am. Chem. Soc. 2011, 133, 5806–5809;
- 6bM. Banerjee, S. Das, M. Yoon, H. J. Choi, M. H. Hyun, S. M. Park, G. Seo, K. Kim, J. Am. Chem. Soc. 2009, 131, 7524–7525;
- 6cF. J. Song, C. Wang, J. M. Falkowski, L. Q. Ma, W. B. Lin, J. Am. Chem. Soc. 2010, 132, 15390–15398.
- 7
- 7aM. Naodovic, H. Yamamoto, Chem. Rev. 2008, 108, 3132–3148;
- 7bA. Fürstner, Chem. Soc. Rev. 2009, 38, 3208–3221;
- 7cS. Díez-González, N. Marion, P. S. Nolan, Chem. Rev. 2009, 109, 3612–3676;
- 7dL. Hao, Z. P. Zhan, Curr. Org. Chem. 2011, 15, 1625–1643.
- 8
- 8aH. Y. Kim, H. J. Shih, W. E. Knabe, K. G. Oh, Angew. Chem. 2009, 121, 7556–7559; Angew. Chem. Int. Ed. 2009, 48, 7420–7423;
- 8bL. C. Wieland, E. M. Vieira, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2009, 131, 570–576;
- 8cY. Yamashita, X. X. Guo, R. Takashita, S. Kobayashi, J. Am. Chem. Soc. 2010, 132, 3262–3263.
- 9
- 9aT. Arai, A. Mishiro, N. Yokoyama, K. Suzuki, H. Sata, J. Am. Chem. Soc. 2010, 132, 5338–5339;
- 9bX. X. Yan, Q. Peng, Y. Zhang, K. Zhang, W. Hong, X. L. Hou, Y. D. Wu, Angew. Chem. 2006, 118, 2013–2017; Angew. Chem. Int. Ed. 2006, 45, 1979–1983;
- 9cN. S. Josephsohn, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2003, 125, 4018–4019.
- 10
- 10aR. P. Lemieux, Acc. Chem. Res. 2001, 34, 845–853;
- 10bJ. Crassous, Chem. Soc. Rev. 2009, 38, 830–845;
- 10cC. He, Y. G. Zhao, D. Guo, Z. H. Lin, C. Y. Duan, Eur. J. Inorg. Chem. 2007, 3451–3463.
- 11
- 11aF. Sladojevich, A. Trabocchi, A. Guarna, D. J. Dixon, J. Am. Chem. Soc. 2011, 133, 1710–1713;
- 11bC. Alemparte, G. Blay, K. A. Jørgensen, Org. Lett. 2005, 7, 4569–4572;
- 11cR. E. Morris, X. H. Bu, Nat. Chem. 2010, 2, 353–361.
- 12Crystal data for Ag-TPHA-1: C97H117N26O7Ag3B3F12Cl3, M=2449.56, cubic, space group I213, yellow block, a=25.381(4) Å, V=16350(5) Å3, Z=4, ρcalcd=0.993 g cm−3, μ(Mo-Kα)=0.462 mm−1, T=180(2) K. 4772 unique reflections [Rint=0.1304]. Final R1 [with I>2σ(I)]=0.0777, wR2 (all data)=0.1844. Crystal data for Ag-TPHA-2: C97H117N26O7Ag3B3F12Cl3, M=2449.56, cubic, space group I213, yellow block, a=25.369(5) Å, V=16328(6) Å3, Z=4, ρcalcd=0.994 g cm−3, μ(Mo-Kα)=0.462 mm−1, T=180(2) K. 4796 unique reflections [Rint=0.1355]. Final R1 [with I>2σ(I)]=0.0726, wR2 (all data)=0.1744.
- 13
- 13aA. F. Wells, 3D Nets and Polyhedra, Wiley-Interscience, New York, 1977;
- 13bG. Pringle, Acta Crystallogr. Sect. B 1972, 28, 2326;
- 13cS. R. Batten, R. Robson, Angew. Chem. 1998, 110, 1558–1595;
10.1002/(SICI)1521-3757(19980605)110:11<1558::AID-ANGE1558>3.0.CO;2-7 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1460–1494.10.1002/(SICI)1521-3773(19980619)37:11<1460::AID-ANIE1460>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 14
- 14aQ. Z. Sun, Y. Bai, G. J. He, C. Y. Duan, Z. H. Lin, Q. J. Meng, Chem. Commun. 2006, 2777–2779;
- 14bY. Bai, C. Y. Duan, P. Cai, D. B. Dang, Q. J. Meng, Dalton Trans. 2005, 2678–2680.
- 15
- 15aD. K. Kondepudi, J. Laudadio, K. Asakura, J. Am. Chem. Soc. 1999, 121, 1448–1451;
- 15bD. K. Kondepudi, R. J. Kaufman, N. Singh, Science 1990, 250, 975–977.
- 16
- 16aL. Perez-Gatcia, D. B. Amabilino, Chem. Soc. Rev. 2007, 36, 941–952;
- 16bD. Guo, K. L. Pang, C. Y. Duan, Y. G. Zhao, Q. J. Meng, Chem. Lett. 2002, 10, 1014–1015.
- 17Crystal data for Ag-1: C97H117N26O7Ag3B3F12Cl3, M=2449.56, Cubic, space group I213, yellow block, a=25.689(1) Å, V=16952(1) Å3, Z=4, ρcald=0.958 g cm−3, μ(Mo-Kα)=0.445 mm−1, T=180(2) K. 4945 unique reflections [Rint=0.1327]. Final R1 [with I>2σ(I)]=0.0710, wR2 (all data)=0.1851. Crystal data for Ag-2: C97H117N26O7Ag3B3F12Cl3, M=2449.56, Cubic, space group I213, yellow block, a=25.574(3) Å, V=16726(3) Å3, Z=4, ρcald=0.971 g cm−3, μ(Mo-Kα)=0.451 mm−1, T=180(2) K. 4758 unique reflections [Rint=0.0947]. Final R1 [with I>2σ(I)]=0.0783, wR2 (all data)=0.1459.
- 18
- 18aD. B. Dang, P. Y. Wu, C. He, Z. Xie, C. Y. Duan, J. Am. Chem. Soc. 2010, 132, 14321–14323;
- 18bJ. Zhang, S. M. Chen, T. Wu, P. Y. Feng, X. H. Bu, J. Am. Chem. Soc. 2008, 130, 12882–12883.
- 19
- 19aJ. M. Longmire, B. Wang, X. M. Zhang, J. Am. Chem. Soc. 2002, 124, 13400–13401;
- 19bC. Nájera, M. D. Retamosa, J. M. Sansano, Org. Lett. 2007, 9, 4025–4028.
- 20Crystal data for Ag-3: C103H116N22O11Ag3B3F12Cl6, M=2634.92, Cubic, space group I213, yellow block, a=25.582(1) Å, V=16743(1) Å3, Z=4, ρcald=1.045 cm−3, μ(Mo-Kα)=0.502 mm−1, T=180(2) K. 4790 unique reflections [Rint=0.0809]. Final R1 [with I>2σ(I)]=0.0866, wR2 (all data)=0.2044.
- 21Q. A. Chen, D. S. Wang, Y. G. Zhou, Chem. Commun. 2010, 46, 4043–4051.
- 22The two dimensional sizes of the molecules of MBA and the product are calculated as 3.7×2.8 and 4.8×5.2 Å2, respectively, possibly allowing the ingress or egress of the molecules of the substrates and the product through the cross section (7.5×8.0 Å2) of the channels within the silver(I) containing MOFs.
- 23
- 23aS. Horike, M. Dinca, K. Tamaki, J. R. Long, J. Am. Chem. Soc. 2008, 130, 5854–5855;
- 23bS. Hasegawa, S. Horike, R. Matsuda, S. Furukawa, K. Mochizuki, Y. Kinoshita, S. Kitagawa, J. Am. Chem. Soc. 2007, 129, 2607–2614.
- 24CCDC 860787 (1), 860788 (2), 860785 (Ag-1), 860786 (Ag-2), and 885237 (Ag-3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.