Synthesis of Fluorinated Tricyclic Scaffolds by Intramolecular [2+2] Photocycloaddition Reactions†
Dr. Diego A. Fort
Lehrstuhl für Organische Chemie I, Technische Universität München, Lichtenbergstr. 4, 85747 Garching (Germany) http://www.oc1.ch.tum.de/home_en/
Search for more papers by this authorDr. Thomas J. Woltering
Discovery Chemistry, PRCB, F. Hoffmann-La Roche AG, Grenzacherstrasse 124, 4070 Basel (Switzerland)
Search for more papers by this authorDr. Matthias Nettekoven
Discovery Chemistry, PRCB, F. Hoffmann-La Roche AG, Grenzacherstrasse 124, 4070 Basel (Switzerland)
Search for more papers by this authorDr. Henner Knust
Discovery Chemistry, PRCB, F. Hoffmann-La Roche AG, Grenzacherstrasse 124, 4070 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thorsten Bach
Lehrstuhl für Organische Chemie I, Technische Universität München, Lichtenbergstr. 4, 85747 Garching (Germany) http://www.oc1.ch.tum.de/home_en/
Lehrstuhl für Organische Chemie I, Technische Universität München, Lichtenbergstr. 4, 85747 Garching (Germany) http://www.oc1.ch.tum.de/home_en/Search for more papers by this authorDr. Diego A. Fort
Lehrstuhl für Organische Chemie I, Technische Universität München, Lichtenbergstr. 4, 85747 Garching (Germany) http://www.oc1.ch.tum.de/home_en/
Search for more papers by this authorDr. Thomas J. Woltering
Discovery Chemistry, PRCB, F. Hoffmann-La Roche AG, Grenzacherstrasse 124, 4070 Basel (Switzerland)
Search for more papers by this authorDr. Matthias Nettekoven
Discovery Chemistry, PRCB, F. Hoffmann-La Roche AG, Grenzacherstrasse 124, 4070 Basel (Switzerland)
Search for more papers by this authorDr. Henner Knust
Discovery Chemistry, PRCB, F. Hoffmann-La Roche AG, Grenzacherstrasse 124, 4070 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thorsten Bach
Lehrstuhl für Organische Chemie I, Technische Universität München, Lichtenbergstr. 4, 85747 Garching (Germany) http://www.oc1.ch.tum.de/home_en/
Lehrstuhl für Organische Chemie I, Technische Universität München, Lichtenbergstr. 4, 85747 Garching (Germany) http://www.oc1.ch.tum.de/home_en/Search for more papers by this authorD.A.F. wishes to acknowledge funding by the Roche Postdoc Fellowship (RPF) Program.
Graphical Abstract
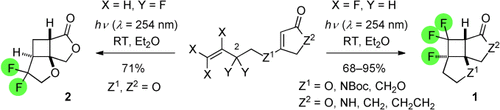
Fabulous Fluorine: The synthesis of fluorinated products 1 and 2 by [2+2] photocycloaddition was readily feasible after optimization of the irradiation conditions. The electron-deficient trifluoroolefin unit reacted intramolecularly to products 1 (nine examples, d.r.>95:5). The reaction was also investigated after modification of position 2 of the side chain both with one or two fluoro substituents (e.g. to yield product 2).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201204080_sm_miscellaneous_information.pdf4.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Hiyama, Organofluorine Compounds, Springer, Berlin, 2010;
- 1bD. O’Hagan, Chem. Soc. Rev. 2008, 37, 308–319;
- 1cK. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, 2006;
10.1002/9780470988589 Google Scholar
- 1dP. Kirsch, Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, Wiley-VCH, Weinheim, 2004.
10.1002/352760393X Google Scholar
- 2
- 2aF. M. D. Ismail, J. Fluorine Chem. 2002, 118, 27–33;
- 2bH.-J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander, M. Stahl, ChemBioChem 2004, 5, 637–643;
- 2cJ. P. Bégué, D. Bonnet-Delpon, J. Fluorine Chem. 2006, 127, 992–1012;
- 2dK. L. Kirk, J. Fluorine Chem. 2006, 127, 1013–1029;
- 2eC. Isanbor, D. O’Hagan, J. Fluorine Chem. 2006, 127, 303–319;
- 2fW. K. Hagmann, J. Med. Chem. 2008, 51, 4359–4369;
- 2gS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 2hA. K. Podichetty, S. Wagner, S. Schröer, A. Faust, O. Schober, M. Schäfers, K. Kopka, G. Haufe, J. Med. Chem. 2009, 52, 3484–3495.
- 3
- 3a Fluorinated Heterocyclic Compounds (Ed.: ), Wiley, Hoboken, 2009;
- 3bS. Fustero, J. F. Sanz-Cervera, J. L. Aceña, M. Sánchez-Roselló, Synlett 2009, 525–549;
- 3cL. E. Zimmer, C. Sparr, R. Gilmour, Angew. Chem. 2011, 123, 12062–12074;
10.1002/ange.201102027 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11860–11871.
- 4Reviews:
- 4aJ. P. Hehn, C. Müller, T. Bach in Handbook of Synthetic Photochemistry (Eds.: ), Wiley-VCH, Weinheim, 2009, pp. 171–215;
10.1002/9783527628193.ch6 Google Scholar
- 4bS. A. Fleming in Molecular and Supramolecular Photochemistry, Vol. 12 (Eds.: ), Marcel Dekker, New York, 2005, pp. 141–160;
- 4cP. Margaretha in Molecular and Supramolecular Photochemistry, Vol. 12 (Eds.: ), Marcel Dekker, New York, 2005, pp. 211–237;
- 4dJ. P. Pete in CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed. ), CRC, Boca Raton, 2004, pp. 71/1–71/14;
- 4eT. Bach, Synthesis 1998, 683–703;
- 4fJ.-P. Pete, Adv. Photochem. 1996, 21, 135–216;
- 4gJ. Mattay, R. Conrads, R. Hoffmann in Methoden der Organischen Chemie (Houben-Weyl) 4te Aufl., Band E 21c (Eds.: ), Thieme, Stuttgart, 1995, pp. 3085–3132;
- 4hM. T. Crimmins, T. L. Reinhold, Org. React. 1993, 44, 297–588.
- 5
- 5aW. P. Dailey, D. M. Lemal, J. Am. Chem. Soc. 1984, 106, 1169–1170;
- 5bR. A. Correa, N. Jing, J. Org. Chem. 1993, 58, 6406–6409.
- 6
- 6aH. Kimoto, K. Takahashi, H. Muramatsu, Bull. Chem. Soc. Jpn. 1980, 53, 764–769;
- 6bD. Andrew, D. J. Hastings, A. C. Weedon, J. Am. Chem. Soc. 1994, 116, 10870–10882.
- 7
- 7aA. J. Wexler, J. S. Swenton, J. Am. Chem. Soc. 1976, 98, 1602–1604;
- 7bA. J. Wexler, R. J. Balchunis, J. S. Swenton, J. Org. Chem. 1984, 49, 2733–2738;
- 7cT. Iwaoka, N. Katagiri, M. Sato, C. Kaneko, Chem. Pharm. Bull. 1992, 40, 2319–2324;
- 7dC. Gauzy, B. Saby, E. Pereira, S. Faure, D. J. Aitken, Synlett 2006, 1394–1398.
- 8A. N. Tkachenko, D. S. Radchenko, P. K. Mykhailiuk, O. O. Grygorenko, I. V. Komarov, Org. Lett. 2009, 11, 5674–5676.
- 9Selected references:
- 9aM. Kemmler, T. Bach, Angew. Chem. 2003, 115, 4973–4975;
10.1002/ange.200352171 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4824–4826;
- 9bM. Kemmler, T. Bach, Eur. J. Org. Chem. 2004, 4582–4595;
- 9cM. Fleck, C. Yang, T. Wada, Y. Inoue, T. Bach, Chem. Commun. 2007, 822–824;
- 9dM. Fleck, T. Bach, Chem. Eur. J. 2010, 16, 6015–6032;
- 9eJ. P. Hehn, D. Gamba-Sánchez, M. Kemmler, M. Fleck, B. Basler, T. Bach, Synthesis 2010, 2308–2312;
- 9fR. Weixler, J. P. Hehn, T. Bach, J. Org. Chem. 2011, 76, 5924–5935.
- 10The electrophilic epoxidation of fluorinated olefins, for example, is notoriously difficult to achieve. For some references, see:
- 10aM. M. Kremlev, I. S. Mazny, Y. L. Yagupolskii, J. Fluorine Chem. 1991, 54, 100;
10.1016/S0022-1139(00)83610-6 Google Scholar
- 10bT. I. Gorbunova, A. Y. Zapevalov, V. I. Saloutin, Russ. Chem. Bull. 1995, 44, 1470–1473;
- 10cA. Arnone, D. D. DesMareau, B. Novo, V. A. Petrov, M. Pregnolato, G. Resnati, J. Org. Chem. 1996, 61, 8805–8810.
- 11Y. Tada, M. Sato, N. Takeno, Y. Nakacho, K. Shigehara, Makromol. Chem. 1993, 194, 2163–2171.
- 12W. R. Li, S. T. Lin, N.-M. Hsu, M. S. Chern, J. Org. Chem. 2002, 67, 4702–4706.
- 13G. R. Waitkins, C. W. Clark, Chem. Rev. 1945, 36, 235–289.
- 14D. I. Schuster, G. N. Lem, A. Kaprinidis, Chem. Rev. 1993, 93, 3–22.
- 15
- 15aH. Meerwein, R. Schmidt, Justus Liebigs Ann. Chem. 1925, 444, 221–238;
- 15bA. Verley, Bull. Soc. Chim. Fr. 1925, 37, 537;
- 15cW. Ponndorf, Angew. Chem. 1926, 39, 138–143;
- 15dJ. Yin, M. A. Huffman, K. M. Conrad, J. D. Armstrong III, J. Org. Chem. 1996, 61, 8805–8810.
- 16For the lack of facial diastereoselectivity in the Paternò–Büchi reaction of α-chiral carbonyl compounds, see:
- 16aS. Jarosz, A. Zamojski, Tetrahedron 1982, 38, 1453–1456;
- 16bS. L. Schreiber, Science 1985, 227, 857–863;
- 16cC. Žagar, H.-D. Scharf, Chem. Ber. 1991, 124, 967–969.
- 17
- 17aA. G. O’Brien, Tetrahedron 2011, 67, 9639–9667;
- 17bA. Mengel, O. Reiser, Chem. Rev. 1999, 99, 1191–1223.