Metal-free Catalytic Olefin Hydrogenation: Low-Temperature H2 Activation by Frustrated Lewis Pairs†
Lutz Greb
Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Department of Chemistry, University of Toronto, Ontario (Canada)
Search for more papers by this authorDr. Pascual Oña-Burgos
Karlsruhe Institute of Technology (KIT), Institute of Inorganic Chemistry, Karlsruhe (Germany)
Search for more papers by this authorBirgitta Schirmer
Organisch-Chemisches Institut, Universität Münster, Münster (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstr. 4, 53115 Bonn (Germany)
Stefan Grimme, Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstr. 4, 53115 Bonn (Germany)
Jan Paradies, Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorProf. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, Ontario (Canada)
Search for more papers by this authorCorresponding Author
Dr. Jan Paradies
Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Stefan Grimme, Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstr. 4, 53115 Bonn (Germany)
Jan Paradies, Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorLutz Greb
Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Department of Chemistry, University of Toronto, Ontario (Canada)
Search for more papers by this authorDr. Pascual Oña-Burgos
Karlsruhe Institute of Technology (KIT), Institute of Inorganic Chemistry, Karlsruhe (Germany)
Search for more papers by this authorBirgitta Schirmer
Organisch-Chemisches Institut, Universität Münster, Münster (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstr. 4, 53115 Bonn (Germany)
Stefan Grimme, Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstr. 4, 53115 Bonn (Germany)
Jan Paradies, Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorProf. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, Ontario (Canada)
Search for more papers by this authorCorresponding Author
Dr. Jan Paradies
Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Stefan Grimme, Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie, Universität Bonn, Beringstr. 4, 53115 Bonn (Germany)
Jan Paradies, Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry, Fritz-Haber Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorThe LGF, FCI, and AvH are gratefully acknowledged by L.G., J.P., and P.O.B. for financial support. J.P. thanks S. Bräse and F. Breher for kind support and fruitful discussions. B.S., S.G., and D.W.S. gratefully acknowledge the financial support from the DFG-SNF 1175 and NSERC of Canada for the award of a Canada Research Chair.
Graphical Abstract
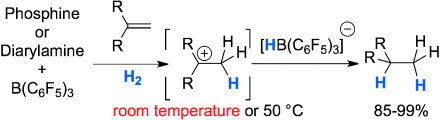
Weak nucleophiles for strong activation: The reversible activation of dihydrogen by an electron-deficient phosphine, (C6F5)PPh2, in combination with the Lewis acid B(C6F5)3 at −80 °C was accomplished. The catalytic hydrogenation of olefins proceeds through protonation and subsequent hydride attack. Electron-deficient phosphines and diarlyamines were demonstrated to be viable Lewis bases for the reaction, thus allowing catalyst loadings of 10 to 5 mol %.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201204007_sm_miscellaneous_information.pdf14.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Sabatier, M. J. Nye, Chem. World 2004, 1, 46–49;
- 1bP. Sabatier, La Catalyse en chimie organique, Paris, Branger, 1913;
- 1cG. Wilkinson, Bull. Soc. Chim. Fr. 1968, 12, 5055–5058.
- 2 Handbook of Homogeneous Hydrogenation (Eds.: ), Wiley-VCH, Weinheim, 2006.
- 3
- 3aR. Noyori, Angew. Chem. 2002, 114, 2108–2123;
10.1002/1521-3757(20020617)114:12<2108::AID-ANGE2108>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2008–2022;10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 3bW. S. Knowles, Angew. Chem. 2002, 114, 2096–2107; Angew. Chem. Int. Ed. 2002, 41, 1998–2007.
- 4for reviews see:
- 4aC. Zheng, S. L. You, Chem. Soc. Rev. 2012, 41, 2498–2518;
- 4bS. G. Ouellet, A. M. Walji, D. W. C. MacMillan, Acc. Chem. Res. 2007, 40, 1327–1339; for recent examples see:
- 4cJ. Paradies, J. F. Schneider, M. B. Lauber, V. Muhr, D. Kratzer, Org. Biomol. Chem. 2011, 9, 4323–4327;
- 4dN. J. A. Martin, X. Cheng, B. List, J. Am. Chem. Soc. 2008, 130, 13862–13863;
- 4eM. Rueping, A. P. Antonchick, T. Theissmann, Angew. Chem. 2006, 118, 6903–6907;
10.1002/ange.200601832 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6751–6755;
- 4fS. G. Ouellet, J. B. Tuttle, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 32–33;
- 4gM. Rueping, E. Sugiono, C. Azap, T. Theissmann, M. Bolte, Org. Lett. 2005, 7, 3781–3783.
- 5
- 5aM. Rubin, T. Schwier, V. Gevorgyan, J. Org. Chem. 2002, 67, 1936–1940;
- 5bJ. M. Blackwell, E. R. Sonmor, T. Scoccitti, W. E. Piers, Org. Lett. 2000, 2, 3921–3923;
- 5cA. Berkefeld, W. E. Piers, M. Parvez, J. Am. Chem. Soc. 2010, 132, 10660–10661;
- 5dW. E. Piers, T. Chivers, Chem. Soc. Rev. 1997, 26, 345–354;
- 5eW. E. Piers, A. J. V. Marwitz, L. G. Mercier, Inorg. Chem. 2011, 50, 12252–12262.
- 6G. C. Welch, R. R. S. Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124–1126.
- 7
- 7aD. W. Stephan, S. Greenberg, T. W. Graham, P. Chase, J. J. Hastie, S. J. Geier, J. M. Farrell, C. C. Brown, Z. M. Heiden, G. C. Welch, M. Ullrich, Inorg. Chem. 2011, 50, 12338–12348;
- 7bD. W. Stephan, G. Erker, Angew. Chem. 2010, 122, 50–81; Angew. Chem. Int. Ed. 2010, 49, 46–76;
- 7cD. W. Stephan, Dalton Trans. 2009, 3129–3136;
- 7dD. W. Stephan, Org. Biomol. Chem. 2008, 6, 1535–1539.
- 8
- 8aP. A. Chase, T. Jurca, D. W. Stephan, Chem. Commun. 2008, 1701–1703;
- 8bP. Spies, S. Schwendemann, S. Lange, G. Kehr, R. Fröhlich, G. Erker, Angew. Chem. 2008, 120, 7654–7657;
10.1002/ange.200801432 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7543–7546;
- 8cP. A. Chase, G. C. Welch, T. Jurca, D. W. Stephan, Angew. Chem. 2007, 119, 8196–8199;
10.1002/ange.200702908 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8050–8053.
- 9
- 9aL. Greb, P. Oña-Burgos, A. Kubas, F. C. Falk, F. Breher, K. Fink, J. Paradies, Dalton Trans. 2012, 41, 9056–9060;
- 9bH. D. Wang, R. Fröhlich, G. Kehr, G. Erker, Chem. Commun. 2008, 5966–5968.
- 10T. Mahdi, Z. M. Heiden, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 4088–4091.
- 11
- 11aV. Sumerin, K. Chernichenko, M. Nieger, M. Leskela, B. Rieger, T. Repo, Adv. Synth. Catal. 2011, 353, 2093–2110;
- 11bV. Sumerin, F. Schulz, M. Nieger, M. Leskela, T. Repo, B. Rieger, Angew. Chem. 2008, 120, 6090–6092;
10.1002/ange.200800935 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6001–6003;
- 11cV. Sumerin, F. Schulz, M. Atsumi, C. Wang, M. Nieger, M. Leskela, T. Repo, P. Pyykko, B. Rieger, J. Am. Chem. Soc. 2008, 130, 14117–14118.
- 12
- 12aC. B. Caputo, S. J. Geier, D. Winkelhaus, N. W. Mitzel, V. N. Vukotic, S. J. Loeb, D. W. Stephan, Dalton Trans. 2012, 41, 2131–2139;
- 12bS. J. Geier, P. A. Chase, D. W. Stephan, Chem. Commun. 2010, 46, 4884–4886;
- 12cS. J. Geier, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 3476–3477.
- 13
- 13aP. A. Chase, A. L. Gille, T. M. Gilbert, D. W. Stephan, Dalton Trans. 2009, 7179–7188;
- 13bD. Holschumacher, C. Taouss, T. Bannenberg, C. G. Hrib, C. G. Daniliuc, P. G. Jones, M. Tamm, Dalton Trans. 2009, 6927–6929;
- 13cP. A. Chase, D. W. Stephan, Angew. Chem. 2008, 120, 7543–7547;
10.1002/ange.200802596 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7433–7437;
- 13dD. Holschumacher, T. Bannenberg, C. G. Hrib, P. G. Jones, M. Tamm, Angew. Chem. 2008, 120, 7538–7542;
10.1002/ange.200802705 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7428–7432.
- 14C. Jiang, D. W. Stephan, Dalton Trans. 2012, DOI: .
- 15The formation of the phosphonium borate salt is additionally facilitated by the increased dielectric constant of the solvent at this temperature. See: I. Fernández, E. Martínez-Viviente, F. Breher, P. S. Pregosin, Chem. Eur. J. 2005, 11, 1495–1506.
- 16M. Ullrich, A. J. Lough, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 52–53.
- 17No reaction was observed when 3 a was reacted with either 1 or 2 and 1/2 in the absence of hydrogen.
- 18This notion is also consistent with the inability to observe the activation of hydrogen in solution by 1/5 even at −90 °C by NMR spectroscopy.
- 19
- 19aA. Stute, G. Kehr, R. Fröhlich, G. Erker, Chem. Commun. 2011, 47, 4288–4290;
- 19bC. Rosorius, G. Kehr, R. Fröhlich, S. Grimme, G. Erker, Organometallics 2011, 30, 4211–4219.
- 20F. C. Falk, R. Fröhlich, J. Paradies, Chem. Commun. 2011, 47, 11095–11097.
- 21
- 21aH. Mayr, A. R. Ofial, J. Phys. Org. Chem. 2008, 21, 584–595;
- 21bT. B. Phan, M. Breugst, H. Mayr, Angew. Chem. 2006, 118, 3954–3959;
10.1002/ange.200600542 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3869–3874;
- 21cH. Mayr, M. Patz, Angew. Chem. 1994, 106, 990–1010; Angew. Chem. Int. Ed. Engl. 1994, 33, 938–957.
- 22H. A. Laub, H. Yamamoto, H. Mayr, Org. Lett. 2010, 12, 5206–5209.
- 23H. Mayr, B. Kempf, A. R. Ofial, Acc. Chem. Res. 2003, 36, 66–77.
- 24H. Mayr, T. Bug, M. F. Gotta, N. Hering, B. Irrgang, B. Janker, B. Kempf, R. Loos, A. R. Ofial, G. Remennikov, H. Schimmel, J. Am. Chem. Soc. 2001, 123, 9500–9512.
- 25
- 25aG. Ménard, D. W. Stephan, Angew. Chem. 2012, 124, 8397–8400;
10.1002/ange.201203362 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 8272–8275;
- 25bX. Zhao, D. W. Stephan, Chem. Sci. 2012, 3, 2123–2132;
- 25cX. Zhao, D. W. Stephan, J. Am. Chem. Soc. 2011, 133, 12448–12450.
- 26No reaction was observed when 3 a was reacted with PhNMe2 or Ph2NMe (7) and 1 in the absence of H2.
- 27T. Voss, T. Mahdi, E. Otten, G. Kehr, R. Fröhlich, D. W. Stephan, G. Erker, Organometallics 2012, 31, 2367–2378.
- 28S. Grimme, J. Chem. Phys. 2006, 124, 034108.
- 29
- 29aS. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104;
- 29bS. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456–1465.
- 30
- 30aF. Eckert, A. Klamt; COSMOtherm, Version C2.1, Release 01.11; COSMOlogic GmbH & Co. KG, Leverkusen, Germany, 2010;
- 30bJ. J. P. Stewart, J. Mol. Model. 2007, 13, 1173–1213;
- 30cF. Eckert, A. Klamt, Aiche J. 2002, 48, 369–385;
- 30dA. Klamt, J. Phys. Chem. 1995, 99, 2224–2235;
- 30eA. Klamt, G. Schüürmann, J. Chem. Soc. Perkin Trans. 2 1993, 799–805.
- 31J. B. Lambert, Y. Zhao, H. W. Wu, J. Org. Chem. 1999, 64, 2729–2736.
- 32B. H. Xu, G. Kehr, R. Fröhlich, B. Wibbeling, B. Schirmer, S. Grimme, G. Erker, Angew. Chem. 2011, 123, 7321–7324; Angew. Chem. Int. Ed. 2011, 50, 7183–7186.
- 33T. A. Rokob, A. Hamza, I. Papai, J. Am. Chem. Soc. 2009, 131, 10701–10710.