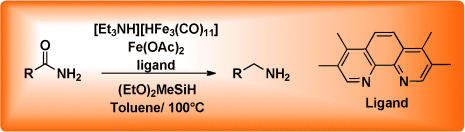
Two Iron Catalysts are Better than One: A General and Convenient Reduction of Aromatic and Aliphatic Primary Amides†
Dr. Shoubhik Das
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorBianca Wendt
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorDr. Konstanze Möller
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorDr. Kathrin Junge
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.deSearch for more papers by this authorDr. Shoubhik Das
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorBianca Wendt
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorDr. Konstanze Möller
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorDr. Kathrin Junge
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.de
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock (Germany) http://www.catalysis.deSearch for more papers by this authorThis work has been funded by the State of Mecklenburg-Western Pomerania, the BMBF, and the DFG (Leibniz-price). We thank Dr. W. Baumann, Dr. C. Fischer, S. Buchholz, S. Schareina, A. Kammer, A. Koch, S. Rossmeisl, and K. Mevius (all at the LIKAT) for their excellent technical and analytical support.
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201108155_sm_miscellaneous_information.pdf190.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Ricci, Modern Amination Methods, Wiley, New York, 2000;
10.1002/9783527613182 Google Scholar
- 1bH. A. Wittcoff, B. G. Reuben, J. S. Plotkin, Industrial Organic Chemicals, 2nd ed., Wiley, New York, 2004.
10.1002/0471651540 Google Scholar
- 2Selected examples:
- 2aT. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev. 2008, 108, 3795;
- 2bO. Y. Lee, K. L. Law, C. Y. Ho, D. Yang, J. Org. Chem. 2008, 73, 8829;
- 2cP. W. Roesky, Angew. Chem. 2009, 121, 4988; Angew. Chem. Int. Ed. 2009, 48, 4892;
- 2dK. Krüger, A. Tillack, M. Beller, ChemSusChem 2009, 2, 715;
- 2eS. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann, M. Beller, Chem. Eur. J. 2011, 17, 4705.
- 3
- 3aS. Enthaler, K. Junge, D. Addis, G. Erre, M. Beller, ChemSusChem 2008, 1, 1006;
- 3bS. Enthaler, D. Addis, K. Junge, G. Erre, M. Beller, Chem. Eur. J. 2008, 14, 9491;
- 3cD. V. Gutsulyak, G. I. Nikonov, Angew. Chem. 2010, 122, 7715;
10.1002/ange.201003069 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7553.
- 4
- 4aT. Gross, A. M. Seayad, M. Ahmad, M. Beller, Org. Lett. 2002, 4, 2055;
- 4bS. Ogo, K. Uehara, T. Abura, S. Fukuzumi, J. Am. Chem. Soc. 2004, 126, 3020.
- 5A. A. Núñez Magro, G. R. Eastham, D. J. Cole-Hamilton, Chem. Commun. 2007, 3154.
- 6J. March, Advanced Organic Chemistry, 4th ed., Wiley, New York, 1992, and references therein.
- 7
- 7aC. Gunanathan, D. Milstein, Angew. Chem. 2008, 120, 8789;
10.1002/ange.200803229 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8661;
- 7bD. Pingen, C. Müller, D. Vogt, Angew. Chem. 2010, 122, 8307;
10.1002/ange.201002583 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8130;
- 7cS. Imm, S. Bähn, L. Neubert, H. Neumann, M. Beller, Angew. Chem. 2010, 122, 8303;
10.1002/ange.201002576 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8126.
- 8
- 8aW. A. Herrmann, J. A. Kulpe, J. Kellner, H. Riepl, H. Bahrmann, W. Konkol, Angew. Chem. 1990, 102, 408; Angew. Chem. Int. Ed. Engl. 1990, 29, 391;
- 8bB. Zimmermann, J. Herwig, M. Beller, Angew. Chem. 1999, 111, 2515;
10.1002/(SICI)1521-3757(19990816)111:16<2515::AID-ANGE2515>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2372.10.1002/(SICI)1521-3773(19990816)38:16<2372::AID-ANIE2372>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 9J. Seyden-Penne, Reductions by Alumino and Borohydrides in Organic Synthesis, 2nd ed., Wiley, New York, 1997.
- 10G. Rothenberg, Catalysis: Concepts and Green Applications, Wiley-VCH, Weinheim, 2008.
10.1002/9783527621866 Google Scholar
- 11J. G. de Vries, C. J. Elsevier, Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, 2007.
- 12
- 12aI. Ojima in The chemistry of organic silicon compounds, Vol. 2 (Eds.: ), Wiley, New York, 1989;
10.1002/0470025107.ch25 Google Scholar
- 12bB. Marciniec, Comprehensive Handbook on Hydrosilylation, Pergamon, Oxford, 1992;
- 12cH. Nishiyama, K. Itoh, Asymmtric hydrosilylation and related reactions in Catalytic Asymmetric Synthesis (Ed.: ), Wiley-VCH, New York, 2000, pp. 111–143;
10.1002/0471721506.ch2 Google Scholar
- 12dD. Addis, S. Das, K. Junge, M. Beller, Angew. Chem. 2011, 123, 6128;
10.1002/ange.201100145 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6004.
- 13
- 13aR. Kuwano, M. Takahashi, Y. Ito, Tetrahedron Lett. 1998, 39, 1017;
- 13bM. Igarashi, T. Fuchikami, Tetrahedron Lett. 2001, 42, 1945;
- 13cT. Ohta, M. Kamiya, M. Nobumoto, K. Kusui, I. Furukawa, Bull. Chem. Soc. Jpn. 2005, 78, 1856;
- 13dY. Motoyama, K. Mitsui, T. Ishihda, H. Nagashima, J. Am. Chem. Soc. 2005, 127, 13150;
- 13eS. Hanada, T. Ishida, Y. Motoyama, H. Nagashima, J. Org. Chem. 2007, 72, 7551;
- 13fN. Sakai, K. Fuhji, T. Konakahara, Tetrahedron Lett. 2008, 49, 6873;
- 13gS. Hanada, E. Tsutsumi, Y. Motoyama, H. Nagashima, J. Am. Chem. Soc. 2009, 131, 15032;
- 13hY. Sunada, H. Kawakami, T. Imaoka, Y. Motoyama, H. Nagashima, Angew. Chem. 2009, 121, 9675;
10.1002/ange.200905025 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9511;
- 13iS. Das, D. Addis, S. Zhou, K. Junge, M. Beller, J. Am. Chem. Soc. 2010, 132, 1770;
- 13jS. Das, D. Addis, K. Junge, M. Beller, Chem. Eur. J. 2011, 17, 12186.
- 14
- 14aS. Hanada, Y. Motoyama, H. Nagashima, Eur. J. Org. Chem. 2008, 4097;
- 14bS. Zhou, K. Junge, D. Addis, S. Das, M. Beller, Org. Lett. 2009, 11, 2461;
- 14cS. Zhou, D. Addis, S. Das, K. Junge, M. Beller, Chem. Commun. 2009, 4883.
- 15For recent examples of Fe-catalyzed reductions see:
- 15aC. P. Casey, H. Guan, J. Am. Chem. Soc. 2007, 129, 5816;
- 15bC. Sui-Seng, F. Freutel, A. J. Lough, R. H. Morris, Angew. Chem. 2008, 120, 954;
10.1002/ange.200705115 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 940;
- 15cK. T. Sylvester, P. J. Chirik, J. Am. Chem. Soc. 2009, 131, 8772;
- 15dA. M. Tondreau, J. M. Darmon, B. M. Wile, S. K. Floyd, E. Lobkovsky, P. J. Chirik, Organometallics 2009, 28, 3928;
- 15eN. Meyer, A. J. Lough, R. H. Morris, Chem. Eur. J. 2009, 15, 5605;
- 15fA. Mikhailine, A. J. Lough, R. H. Morris, J. Am. Chem. Soc. 2009, 131, 1394;
- 15gC. P. Casey, H. Guan, J. Am. Chem. Soc. 2009, 131, 2499;
- 15hS. Zhou, S. Fleischer, K. Junge, S. Das, D, Addis, M. Beller, Angew. Chem. 2010, 122, 8298–8302; Angew. Chem. Int. Ed. 2010, 49, 8121–8125;
- 15iG. Wienhöfer, I. Sorribes, A. Boddien, F. Westerhaus, K. Junge, H. Junge, R. Llusar, M. Beller, J. Am. Chem. Soc. 2011, 133, 12875.
- 16For recent reviews and highlights on using iron catalysts see:
- 16aC. Bolm, J. Legros, J. L. Paith, L. Zani, Chem. Rev. 2004, 104, 6217;
- 16bS. Enthaler, K. Junge, M. Beller, Angew. Chem. 2008, 120, 3363;
10.1002/ange.200800012 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3317;
- 16cA. Correa, O. G. Mancheño, C. Bolm, Chem. Soc. Rev. 2008, 37, 1108;
- 16dE. B. Bauer, Curr. Org. Chem. 2008, 12, 1341;
- 16eW. M. Czaplik, M. Mayer, A. J. von Wangelin, Angew. Chem. 2009, 121, 616; Angew. Chem. Int. Ed. 2009, 48, 607;
- 16fK. Junge, K. Schröder, M. Beller, Chem. Commun. 2011, 47, 4849.
- 17For Fe-catalyzed hydrosilylations see:
- 17aN. S. Shaikh, K. Junge, M. Beller, Org. Lett. 2007, 9, 5429;
- 17bH. Nishiyama, A. Furuta, Chem. Commun. 2007, 760;
- 17cN. S. Shaikh, S. Enthaler, K. Junge, M. Beller, Angew. Chem. 2008, 120, 2531;
10.1002/ange.200705624 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2497;
- 17dA. M. Tondreau, E. Lobkovsky, P. J. Chirik, Org. Lett. 2008, 10, 2789;
- 17eA. Furuta, H. Nishiyama, Tetrahedron Lett. 2008, 49, 110;
- 17fB. K. Langlotz, H. Wadepohl, L. H. Gade, Angew. Chem. 2008, 120, 4748;
10.1002/ange.200801150 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4670; Ref. [16f].
- 18For catalytic hydrosilylations of nitriles see:
- 18aT. Murai, T. Sakane, S. Kato, J. Org. Chem. 1990, 55, 449;
- 18bA. M. Caporusso, N. Panziera, P. Pertici, E. Pitzalis, P. Salvadori, G. Vitulli, G. Martra, J. Mol. Catal. A 1999, 150, 275.