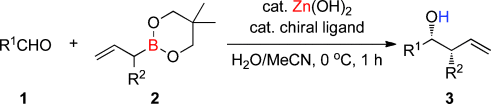Chiral Zinc-Catalyzed Asymmetric α-Alkylallylation and α-Chloroallylation of Aldehydes†
Corresponding Author
Prof. Dr. Shū Kobayashi
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)Search for more papers by this authorToshimitsu Endo
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)
Search for more papers by this authorDr. Masaharu Ueno
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shū Kobayashi
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)Search for more papers by this authorToshimitsu Endo
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)
Search for more papers by this authorDr. Masaharu Ueno
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo, 113-0033 (Japan)
Search for more papers by this authorThis work was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS), ERATO (JST), NEDO, and GCOE. We would also like to thank Mr. Takeshi Naito (The University of Tokyo) for the X-ray crystal-structure analysis.
Graphical Abstract
Two birds with one stone: In the presence of Zn(OH)2 and a chiral bipyridine ligand, racemic α-substituted allylboronates 2 reacted with aldehydes 1 (see scheme) exclusively in an α-addition fashion to afford various homoallylic alcohols 3 bearing two neighboring stereogenic centers in high yields with high diastereo- and enantioselectivities.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201106433_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Catalytic Asymmetric Synthesis (Ed.: ), Wiley, New York, 2010.
10.1002/9780470584248 Google Scholar
- 2
- 2aH. Lachance, D. G. Hall, Org. React. 2008, 73, 1;
- 2bS. E. Denmark, J. Fu, Chem. Rev. 2003, 103, 2763;
- 2cS. E. Denmark, N. G. Almstead in Modern Carbonyl Chemistry (Ed.: ), Wiley-VCH, Weinheim, 2000, chap. 10, pp. 299–402;
10.1002/9783527613267.ch10 Google Scholar
- 2dS. R. Chemler, W. R. Roush in Modern Carbonyl Chemistry (Ed.: ), Wiley-VCH, Weinheim, 2000, chap. 11, pp. 403–490.
10.1002/9783527613267.ch11 Google Scholar
- 3
- 3aA. L. Costa, M. G. Piazza, E. Tagliavini, C. Trombini, A. Umani-Rochi, J. Am. Chem. Soc. 1993, 115, 7001;
- 3bG. E. Keck, K. H. Tarbet, L. S. Geraci, J. Am. Chem. Soc. 1993, 115, 8467;
- 3cA. Yanagisawa, H. Nakashima, A. Ishiba, H. Yamamoto, J. Am. Chem. Soc. 1996, 118, 4723.
- 4
- 4aK. Furuta, M. Mouri, H. Yamamoto, Synlett 1991, 561;
- 4bK. Ishihara, M. Mouri, Q. Z. Gao, T. Maruyama, K. Furuta, H. Yamamoto, J. Am. Chem. Soc. 1993, 115, 11490;
- 4cD. R. Gauthier, E. M. Carreira, Angew. Chem. 1996, 108, 2521;
10.1002/ange.19961082018 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2363;
- 4dM. Wadamoto, N. Ozasa, A. Yanagisawa, H. Yamamoto, J. Org. Chem. 2003, 68, 5593;
- 4eS. E. Denmark, J. Fu, J. Am. Chem. Soc. 2001, 123, 9488;
- 4fS. E. Denmark, J. Fu, Chem. Commun. 2003, 167;
- 4gA. V. Malkov, L. Dufkova, L. Farrugia, P. Kocovsky, Angew. Chem. 2003, 115, 3802;
10.1002/ange.200351737 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3674;
- 4hA. V. Malkov, M. Bell, F. Castelluzzo, P. Kocovsky, Org. Lett. 2005, 7, 3219.
- 5For other representative catalytic asymmetric allylations of aldehydes, see:
- 5aC. Chen, K. Tagami, Y. Kishi, J. Org. Chem. 1995, 60, 5386;
- 5bA. Fürstner, N. Shi, J. Am. Chem. Soc. 1996, 118, 12349;
- 5cM. Bandini, P. G. Cozzi, P. Melchiorre, A. Umani-Rochi, Angew. Chem. 1999, 111, 3558;
10.1002/(SICI)1521-3757(19991115)111:22<3558::AID-ANGE3558>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3357;10.1002/(SICI)1521-3773(19991115)38:22<3357::AID-ANIE3357>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 5dH. W. Choi, K. Nakajima, D. Demeke, F. A. Kang, H. S. Jun, Z. K. Wan, Y. Kishi, Org. Lett. 2002, 4, 4431;
- 5eA. Fürstner, M. Wuchrer, Chem. Eur. J. 2006, 12, 76;
- 5fG. Xia, H. Yamamoto, J. Am. Chem. Soc. 2006, 128, 2554;
- 5gI. S. Kim, M.-Y. Ngai, M. J. Krische, J. Am. Chem. Soc. 2008, 130, 14891;
- 5hI. S. Kim, S. B. Han, M. J. Krische, J. Am. Chem. Soc. 2008, 130, 6340.
- 6
- 6aJ. W. J. Kennedy, D. G. Hall, J. Am. Chem. Soc. 2002, 124, 11586;
- 6bT. Ishiyama, T.-A. Ahiko, N. Miyaura, J. Am. Chem. Soc. 2002, 124, 12414;
- 6cV. Rauniyar, D. G. Hall, J. Am. Chem. Soc. 2004, 126, 4518;
- 6dS. H. Yu, M. J. Ferguson, R. McDonald, D. G. Hall, J. Am. Chem. Soc. 2005, 127, 12808;
- 6eV. Rauniyar, D. G. Hall, Angew. Chem. 2006, 118, 2486;
10.1002/ange.200504432 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2426;
- 6fD. G. Hall, Synlett 2007, 1644;
- 6gV. Rauniyar, H. Zhai, D. G. Hall, J. Am. Chem. Soc. 2008, 130, 8481;
- 6hV. Rauniyar, D. G. Hall, J. Org. Chem. 2009, 74, 4236;
- 6iP. Jain, J. C. Antilla, J. Am. Chem. Soc. 2010, 132, 11884;
- 6jR. Wada, K. Oisaki, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2004, 126, 8910;
- 6kS. Lou, P. N. Moquist, S. E. Schaus, J. Am. Chem. Soc. 2006, 128, 12660;
- 6lD. S. Barnett, P. N. Moquist, S. E. Schaus, Angew. Chem. 2009, 121, 8835;
10.1002/ange.200904715 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8679;
- 6mP. Zhang, J. P. Morken, J. Am. Chem. Soc. 2009, 131, 12550.
- 7Numerous studies have been done in the area of carbonyl allylboration, including asymmetric reactions with chiral boron reagents (using stoichiometric amounts of chiral sources). For selected papers, see:
- 7aR. W. Hoffmann, Pure Appl. Chem. 1990, 62, 1993;
- 7bR. W. Hoffmann, Pure Appl. Chem. 1988, 60, 123;
- 7cH. C. Brown, U. S. Racherla, P. J. Pellechia, J. Org. Chem. 1990, 55, 1868;
- 7dP. V. Ramachandran, Aldrichimica Acta 2002, 35, 23. See also Ref. [6f] and references cited therein. More recently, Ir-catalyzed, enantioselective carbonyl allylation using allyl acetates as allyl metal surrogates has been reported. Selected papers:
- 7eSee Ref. [5g];
- 7fI. S. Kim, S. B. Han, M. J. Krische, J. Am. Chem. Soc. 2009, 131, 2514;
- 7gS. B. Han, X. Gao, J. Krische, J. Am. Chem. Soc. 2010, 132, 9153, and references therein.
- 8
- 8aS. Kobayashi, T. Endo, U. Schneider, M. Ueno, Chem. Commun. 2010, 46, 1260; Cf;
- 8bM. Fujita, T. Nagano, U. Schneider, T. Hamada, C. Ogawa, S. Kobayashi, J. Am. Chem. Soc. 2008, 130, 2914.
- 9
- 9aC. Bolm, M. Zehnder, D. Bur, Angew. Chem. 1990, 102, 206; Angew. Chem. Int. Ed. Engl. 1990, 29, 205;
- 9bC. Bolm, M. Ewald, M. Felder, G. Schlingloff, Chem. Ber. 1992, 125, 1169;
- 9cS. Ishikawa, T. Hamada, K. Manabe, S. Kobayashi, Synthesis 2005, 2176.
- 10S. Hu, S. Jayaraman, A. C. Oehlschlager, J. Org. Chem. 1999, 64, 3719.
- 11
- 11aI. Paterson, E. A. Anderson, S. M. Dalby, O. Loiseleur, Org. Lett. 2005, 7, 4121;
- 11bA. Fürstner, M. D. B. Fenster, B. Fasching, C. Godbout, K. Radkowski, Angew. Chem. 2006, 118, 5636;
10.1002/ange.200601655 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5510;
- 11cI. Paterson, E. A. Anderson, S. M. Dalby, J. H. Lim, O. Loiseleur, P. Maltas, C. Moessner, Pure Appl. Chem. 2007, 79, 667.
- 12Although direct observation of the allylzinc species with chiral ligand 4 has not yet been successful, we could detect some allylzinc species with dmp by ESI mass spectrometry.
- 13We used methyl pyruvate instead of an aldehyde in the initial kinetic studies, because the reactions with aldehydes were too fast to conduct simple kinetic experiments. While we have already confirmed similarity between aldehydes and methyl pyruvate in this Zn-catalyzed allylation, further kinetic studies using aldehydes are in progress.
- 14CCDC 827315 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15H.-L. Kwong, K.-M. Lau, W.-S. Lee, W.-T. Wong, New J. Chem. 1999, 23, 629.
- 16While a similar structure was observed in the Cu2+-4 complex, two hydroxy groups coordinate to the metal in a Sc3+-4 complex;
- 16aS. Ishikawa, T. Hamada, K. Manabe, S. Kobayashi, J. Am. Chem. Soc. 2004, 126, 12236;
- 16bM. Kokubo, T. Naito, S. Kobayashi, Chem. Lett. 2009, 38, 904.
- 17S. J. Archibald in Comprehensive Coordination Chemistry II, Vol. 6 (Ed.: ), Elsevier, London, 2004, chap. 6.8, pp. 1147–1251.
10.1016/B0-08-043748-6/05130-6 Google Scholar
- 18M. Kokubo, T. Naito, S. Kobayashi, Tetrahedron 2010, 66, 1111.
- 19Examples of catalytic, highly enantioselective allylation of aldehydes in aqueous media are very rare. See S. Kobayashi, N. Aoyama, K. Manabe, Chirality 2003, 15, 124, and references therein.
- 20
- 20aT. Krämer, J.-R. Schwark, D. Hoppe, Tetrahedron Lett. 1989, 30, 7037;
- 20bJ. A. Marshall, K. W. Hinkle, J. Org. Chem. 1995, 60, 1920;
- 20cD. J. Hallett, E. J. Thomas, Tetrahedron: Asymmetry 1995, 6, 2575;
- 20dG. W. Bradley, D. J. Hallett, E. J Thomas, Tetrahedron: Asymmetry 1995, 6, 2579;
- 20eA. Yanagisawa, S. Habaue, H. Yamamoto, J. Am. Chem. Soc. 1991, 113, 8955;
- 20fA. Yanagisawa, S. Habaue, K. Yasue, H. Yamamoto, J. Am. Chem. Soc. 1994, 116, 6130;
- 20gY. Yamamoto, K. Maruyama, J. Org. Chem. 1983, 48, 1564;
- 20hH. Miyabe, Y. Yamaoka, T. Naito, Y. Takemoto, J. Org. Chem. 2003, 68, 6745.
- 21
- 21aH. Ito, S. Ito, Y. Sasaki, K. Matsuura, M. Sawamura, J. Am. Chem. Soc. 2007, 129, 14856;
- 21bA. Guzman-Martinez, A. H. Hoveyda, J. Am. Chem. Soc. 2010, 132, 10634;
- 21cX. Gao, D. G. Hall, J. Am. Chem. Soc. 2003, 125, 9308;
- 21dN. F. Pelz, A. R. Woodward, H. E. Burks, J. D. Sieber, J. P. Morken, J. Am. Chem. Soc. 2004, 126, 16328;
- 21eM. Gerdin, C. Moberg, Adv. Synth. Catal. 2005, 347, 749;
- 21fL. Carosi, D. G. Hall, Angew. Chem. 2007, 119, 6017;
10.1002/ange.200700975 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5913;
- 21gF. Peng, D. G. Hall, Tetrahedron Lett. 2007, 48, 3305; See also:
- 21hG. Y. Fang, V. K. Aggarwal, Angew. Chem. 2007, 119, 363; Angew. Chem. Int. Ed. 2007, 46, 359, and references therein.