High Stokes Shift Anilido-Pyridine Boron Difluoride Dyes†
Juan F. Araneda
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Search for more papers by this authorCorresponding Author
Warren E. Piers
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/Search for more papers by this authorCorresponding Author
Belinda Heyne
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/Search for more papers by this authorMasood Parvez
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Search for more papers by this authorRobert McDonald
Department of Chemistry, University of Alberta, 11227 Saskatchewan Drive, Edmonton, Alberta, T6G 2G2 (Canada)
Search for more papers by this authorJuan F. Araneda
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Search for more papers by this authorCorresponding Author
Warren E. Piers
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/Search for more papers by this authorCorresponding Author
Belinda Heyne
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/Search for more papers by this authorMasood Parvez
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 (Canada) http://www.chem.ucalgary.ca/research/groups/wpiers/
Search for more papers by this authorRobert McDonald
Department of Chemistry, University of Alberta, 11227 Saskatchewan Drive, Edmonton, Alberta, T6G 2G2 (Canada)
Search for more papers by this authorFunding for this work was provided by the NSERC of Canada.
Graphical Abstract
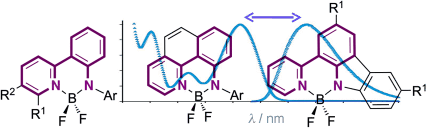
To dye for: Three related families of fluorescent dyes, rigidified by boron difluoride and based on bidentate anilido-pyridyl donor ligands, are reported (see picture). The dyes show quantum yields up to 0.75 and improved Stokes shifts of >100 nm relative to the widely employed BODIPY family of dyes. Furthermore, the new dyes are exceptionally photostable and membrane-specific.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105228_sm_miscellaneous_information.pdf14 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. R. Whittell, M. D. Hager, U. S. Schubert, I. Manners, Nat. Mater. 2011, 10, 176;
- 1bA. Kaeser, A. P. H. J. Schenning, Adv. Mater. 2010, 22, 2985;
- 1cL. Maretti, P. S. Billone, Y. Liu, J. C. Scaiano, J. Am. Chem. Soc. 2009, 131, 13972.
- 2
- 2aM. S. T. Goncalves, Chem. Rev. 2008, 108, 190;
- 2bJ. M. Baumes, J. J. Gassensmith, J. Giblin, J.-J. Lee, A. G. White, W. J. Culligan, W. M. Leevy, M. Kuno, B. D. Smith, Nat. Chem. 2010, 2, 1025;
- 2cR. P. Haugland, Handbook of Molecular Probes and Research Products, 9th ed., Molecular Probes, OR, 2002.
- 3
- 3aJ.-H. Yum, P. Chen, M. Grätzel, M. K. Nazeeruddin, ChemSusChem 2008, 1, 699;
- 3bD. Kumaresan, R. P. Thummel, T. Bura, G. Ulrich, R. Ziessel, Chem. Eur. J. 2009, 15, 6335.
- 4
- 4aA. Loudet, K. Burgess, Chem. Rev. 2007, 107, 4891;
- 4bR. Ziessel, G. Ulrich, A. Harriman, New J. Chem. 2007, 31, 496;
- 4cG. Ulrich, R. Ziessel, A. Harriman, Angew. Chem. 2008, 120, 1202;
10.1002/ange.200702070 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1184.
- 5T. E. Wood, A. Thompson, Chem. Rev. 2007, 107, 1831.
- 6A. Burghart, L. H. Thoresen, J. Chen, K. Burgess, F. Bergstrom, L. B.-A. Johansson, Chem. Commun. 2000, 2203.
- 7L. Bourget-Merle, M. F. Lappert, J. R. Severn, Chem. Rev. 2002, 102, 3031.
- 8B. Qian, S. W. Baek, M. R. Smith III, Polyhedron 1999, 18, 2405.
- 9P. G. Hayes, G. C. Welch, D. J. H. Emslie, C. L. Noack, W. E. Piers, M. Parvez, Organometallics 2003, 22, 1577.
- 10
- 10aY. Ren, X. M. Liu, H. Xia, L. Ye, Y. Mu, Eur. J. Inorg. Chem. 2007, 1808;
- 10bX. M. Liu, Y. Ren, H. Xia, X. Fan, Y. Mu, Inorg. Chim. Acta 2010, 363, 1441.
- 11NN bidentate ligands related to IV:
- 11aQ. D. Liu, M. S. Mudadu, R. Thummel, Y. Tao, S. N. Wang, Adv. Funct. Mater. 2005, 15, 143;
- 11bS. B. Cortright, J. N. Johnston, Angew. Chem. 2002, 114, 355;
10.1002/1521-3757(20020118)114:2<355::AID-ANGE355>3.0.CO;2-E Google ScholarAngew. Chem. Int. Ed. 2002, 41, 345;10.1002/1521-3773(20020118)41:2<345::AID-ANIE345>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 11cS. B. Cortright, J. C. Huffman, R. A. Yoder, J. N. Coalter, J. N. Johnston, Organometallics 2004, 23, 2238.
- 12
- 12aN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457;
- 12bA. Suzuki, Angew. Chem. 2011, 123, 6854; Angew. Chem. Int. Ed. 2011, 50, 6722.
- 13
- 13aJ. P. Wolfe, S. Wagaw, S. L. Buchwald, J. Am. Chem. Soc. 1996, 118, 7215;
- 13bM. S. Driver, J. F. Hartwig, J. Am. Chem. Soc. 1996, 118, 7217.
- 14
- 14aE. Negishi, A. O. King, N. Okukado, J. Org. Chem. 1977, 42, 1821;
- 14bE. Negishi, Angew. Chem. 2011, 123, 6870;
10.1002/ange.201101380 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6738.
- 15B. M. Coleridge, C. S. Bello, D. H. Ellenberger, A. Leitner, Tetrahedron Lett. 2010, 51, 357.
- 16Crystal data for 1 a: C17H13BF2N2, MW=294.10, monoclinic, P21/c, a=11.2101(4), b=7.3817(4), c=17.1219(9) Å, α=90, β=102.339(3), γ=90°, V=1384.10(11) Å3, Z=4, ρ=1.411 g cm−3, MoKα radiation, λ=0.71073 Å, T=173(2) K, 4290 measured reflection, 3143 unique, min/max transmission=0.9839 and 0.9939, R1 (I>2σ)=0.0586, wR2=0.1152, GoF=1.095, No. of parameters=195, final difference map within +0.323 and −0.258 e Å3. Crystal data for 2 a: C19H13BF2N2, MW=318.12, monoclinic, P21/c, a=7.4793(2), b=20.1482(6), c=10.2383(3) Å, α=90, β=111.0840(3), γ=90°, V=1439.57(7) Å3, Z=4, ρ=1.468 g cm−3, MoKα radiation, λ=0.71073 Å, T=173(2) K, 12 783 measured reflection, 3313 unique, min/max transmission=0.9534 and 0.9823, R1 (I>2σ)=0.0338, wR2=0.0948, GoF=1.048, No. of parameters=217, final difference map within +0.283 and −0.206 e Å3. Crystal data for 3 b: C26H29BCl2F2N2, MW=489.22, monoclinic, P21/m, a=11.4796(5), b=6.9227(4), c=15.4393(5) Å, α=90, β=97.296(3), γ=90°, V=1217.02(10) Å3, Z=2, ρ=1.335 g cm−3, MoKα radiation, λ=0.71073 Å, T=173(2) K, 4108 measured reflection, 2965 unique, min/max transmission=0. 9650 and 0.9209 R1 (I>2σ)=0.0746, wR2=0.1721, GoF=1.057, No. of parameters=208, final difference map within +0.726 and −0.767 e Å3. CCDC 836295, 836296, 836297, 836298, 836299, 836290, 836291 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17K. D. Conroy, W. E. Piers, M. Parvez, J. Organomet. Chem. 2008, 693, 834.
- 18
- 18aW. Qin, M. Baruah, M. Van der Auweraer, F. C. De Schryver, N. L. Boens, J. Phys. Chem. A 2005, 109, 7371;
- 18bW. Qin, T. Rohand, M. Baruah, A. Stefan, M. V. der Auweraer, W. Dehaen, N. Boens, Chem. Phys. Lett. 2006, 420, 562;
- 18cA. Filarowski, M. Kluba, K. Cieslik-Boczula, A. Koll, A. Kochel, L. Pandey, W. M. De Borggraeve, M. Van der Auweraer, J. Catalan, N. Boens, Photochem. Photobiol. Sci. 2010, 9, 996.
- 19J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 4th ed., Springer, 2006.
- 20
- 20aJ. Karolin, L. B. A. Johansson, L. Strandberg, T. Ny, J. Am. Chem. Soc. 1994, 116, 7801;
- 20bI. D. Johnson, H. C. Kang, R. P. Haugland, Anal. Biochem. 1991, 198, 228;
- 20cM. Dahim, N. K. Mizuno, X.-M. Li, W. E. Momsen, M. M. Momsen, H. L. Brockman, Biophys. J. 2002, 83, 1511;
- 20dI. A. Boldyrev, X. Zhai, M. M. Momsen, H. L. Brockman, R. E. Brown, J. G. Molotkovsky, J. Lipid Res. 2007, 48, 1518.
- 21B. Heyne, D. Brault, M. P. Fontaine-Aupart, S. Kohnen, F. Tfibel, A. Mouithys-Mickalad, G. Deby-Dupont, P. Hans, M. Hoebeke, Biochim. Biophys. Acta Gen. Subj. 2005, 1724, 100.
- 22
- 22aK. Krumova, G. Cosa, J. Am. Chem. Soc. 2010, 132, 17560;
- 22bM. F. Debets, S. S. van Berkel, J. Dommerholt, A. J. Dirks, F. P. J. T. Rutjes, F. L. van Delft, Acc. Chem. Res. 2011, 44, 805-815;
- 22cE. Saxon, C. R. Bertozzi, Science 2000, 287, 2007.