Active-Metal Template Synthesis of a Molecular Trefoil Knot†
Dr. Perdita E. Barran
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorHarriet L. Cole
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorDr. Stephen M. Goldup
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorCorresponding Author
Prof. David A. Leigh
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.netSearch for more papers by this authorDr. Paul R. McGonigal
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorDr. Mark D. Symes
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorJhenyi Wu
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorMichael Zengerle
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorDr. Perdita E. Barran
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorHarriet L. Cole
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorDr. Stephen M. Goldup
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorCorresponding Author
Prof. David A. Leigh
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.netSearch for more papers by this authorDr. Paul R. McGonigal
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorDr. Mark D. Symes
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorJhenyi Wu
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorMichael Zengerle
School of Chemistry, The University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK) http://www.catenane.net
Search for more papers by this authorWe thank the EPSRC National Mass Spectrometry Service Centre (Swansea (UK)) for high-resolution mass spectrometry. This work was supported by the EPSRC.
Graphical Abstract
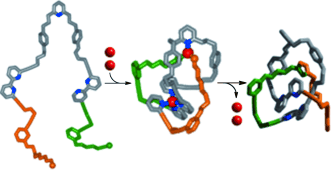
Tying the knot: The marriage of catalysis and coordination chemistry enables two CuI ions (red; see picture) to work in partnership for the synthesis of a molecular trefoil knot. One ion entangles an acyclic building block to create a loop in the ligand, and the other gathers the ligand's reactive end-groups, threads the loop, and catalyzes the covalent capture of the knotted architecture by an alkyne–azide “click” reaction.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105012_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. B. Amabilino, J. F. Stoddart, Chem. Rev. 1995, 95, 2725–2828;
- 1b Molecular Catenanes, Rotaxanes and Knots: A Journey Through the World of Molecular Topology (Eds.: ), Wiley-VCH, Weinheim, 1999;
- 1cG. A. Breault, C. A. Hunter, P. C. Mayers, Tetrahedron 1999, 55, 5265–5293;
- 1dL. Raehm, D. G. Hamilton, J. K. M. Sanders, Synlett 2002, 1743–1761;
- 1eK. Kim, Chem. Soc. Rev. 2002, 31, 96–107;
- 1fE. R. Kay, D. A. Leigh, Top. Curr. Chem. 2005, 262, 133–177;
- 1gH. Tian, Q. C. Wang, Chem. Soc. Rev. 2006, 35, 361–374;
- 1hA. Bogdan, Y. Rudzevich, M. O. Vysotsky, V. Böhmer, Chem. Commun. 2006, 2941–2952;
- 1iJ. R. Nitschke, Acc. Chem. Res. 2007, 40, 103–112;
- 1jS. J. Loeb, Chem. Soc. Rev. 2007, 36, 226–235;
- 1kJ. A. Faiz, V. Heitz, J.-P. Sauvage, Chem. Soc. Rev. 2009, 38, 422–442;
- 1lK. M. Mullen, P. D. Beer, Chem. Soc. Rev. 2009, 38, 1701–1713;
- 1mJ. J. Gassensmith, J. M. Baumes, B. D. Smith, Chem. Commun. 2009, 6329–6338;
- 1nP. Gaviña, S. Tatay, Curr. Org. Synth. 2010, 7, 24–43;
- 1oD.-H. Qu, H. Tian, Chem. Sci. 2011, 2, 1011–1015.
- 2For reviews on molecular trefoil knots see:
- 2aC. Dietrich-Buchecker, B. X. Colasson, J.-P. Sauvage, Top. Curr. Chem. 2005, 249, 261–283;
- 2bO. Lukin, F. Vögtle, Angew. Chem. 2005, 117, 1480–1501;
10.1002/ange.200460312 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1456–1477;
- 2cE. E. Fenlon, Eur. J. Org. Chem. 2008, 5023–5035;
- 2dR. S. Forgan, J.-P. Sauvage, J. F. Stoddart, Chem. Rev. 2011, DOI: .
- 3
- 3aC. C. Adams, The Knot Book, American Mathematical Society, USA, 2004;
- 3b Handbook of Knot Theory (Eds.: ), Elsevier, Amsterdam, 2005.
- 4
- 4aL. F. Liu, R. E. Depew, J. C. Wang, J. Mol. Biol. 1976, 106, 439–452;
- 4bL. F. Liu, C. C. Liu, B. M. Alberts, Cell 1980, 19, 697–707;
- 4cM. A. Krasnow, A. Stasiak, S. J. Spengler, F. Dean, T. Koller, N. R. Cozzarelli, Nature 1983, 304, 559–560.
- 5
- 5aO. Nureki, M. Shirouzu, K. Hashimoto, R. Ishitani, T. Terada, M. Tamakoshi, T. Oshima, M. Chijimatsu, K. Takio, D. G. Vassylyev, T. Shibata, Y. Inoue, S. Kuramitsu, S. Yokoyama, Acta Crystallogr. Sect. D 2002, 58, 1129–1137;
10.1107/S0907444902006601 Google Scholar
- 5bJ. R. Wagner, J. S. Brunzelle, K. T. Forest, R. D. Vierstra, Nature 2005, 438, 325–331.
- 6M. Schappacher, A. Deffieux, Angew. Chem. 2009, 121, 6044–6047;
10.1002/ange.200900704 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5930–5933.
- 7
- 7aC. O. Dietrich-Buchecker, J.-P. Sauvage, Angew. Chem. 1989, 101, 192–194; Angew. Chem. Int. Ed. Engl. 1989, 28, 189–192;
- 7bC. O. Dietrich-Buchecker, J. Guilhem, C. Pascard, J.-P. Sauvage, Angew. Chem. 1990, 102, 1202–1204; Angew. Chem. Int. Ed. Engl. 1990, 29, 1154–1156.
- 8P. R. Ashton, O. A. Matthews, S. Menzer, F. M. Raymo, N. Spencer, J. F. Stoddart, D. J. Williams, Liebigs Ann./Recl. 1997, 2485–2494.
- 9
- 9aJ. E. Mueller, S. M. Du, N. C. Seeman, J. Am. Chem. Soc. 1991, 113, 6306–6308;
- 9bS. M. Du, N. C. Seeman, J. Am. Chem. Soc. 1992, 114, 9652–9655;
- 9cS. M. Du, N. C. Seeman, Biopolymers 1994, 34, 31–37.
- 10
- 10aO. Safarowsky, M. Nieger, R. Fröhlich, F. Vögtle, Angew. Chem. 2000, 112, 1699–1701;
10.1002/(SICI)1521-3757(20000502)112:9<1699::AID-ANGE1699>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1616–1618;10.1002/(SICI)1521-3773(20000502)39:9<1616::AID-ANIE1616>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 10bM. Feigel, R. Ladberg, S. Engels, R. Herbst-Irmer, R. Fröhlich, Angew. Chem. 2006, 118, 5827–5831;
10.1002/ange.200601111 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5698–5702;
- 10cJ. Brüggemann, S. Bitter, S. Müller, W. M. Müller, U. Müller, N. M. Maier, W. Lindner, F. Vögtle, Angew. Chem. 2007, 119, 258–263;
10.1002/ange.200601938 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 254–259.
- 11
- 11aG. Rapenne, C. Dietrich-Buchecker, J.-P. Sauvage, J. Am. Chem. Soc. 1999, 121, 994–1001;
- 11bH. Adams, E. Ashworth, G. A. Breault, J. Guo, C. A. Hunter, P. C. Mayers, Nature 2001, 411, 763;
- 11cE. E. Fenlon, Nat. Chem. 2010, 2, 156–157;
- 11dJ. Guo, P. C. Mayers, G. A. Breault, C. A. Hunter, Nat. Chem. 2010, 2, 218–222.
- 12For studies towards the synthesis of trefoil knots using covalent scaffolds as chemical templates, see:
- 12aC. R. Woods, M. Benaglia, S. Toyota, K. Hardcastle, J. S. Siegel, Angew. Chem. 2001, 113, 771–773;
10.1002/1521-3757(20010216)113:4<771::AID-ANGE7710>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 749–751;10.1002/1521-3773(20010216)40:4<749::AID-ANIE7490>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 12bE. E. Fenlon, B. R. Ito, Eur. J. Org. Chem. 2008, 3065–3068;
- 12cK. I. Arias, E. Zysman-Colman, J. C. Loren, A. Linden, J. S. Siegel, Chem. Commun. 2011, 47, 9588–9590.
- 13
- 13aV. Aucagne, K. D. Hänni, D. A. Leigh, P. J. Lusby, D. B. Walker, J. Am. Chem. Soc. 2006, 128, 2186–2187;
- 13bS. Saito, E, Takahashi, K. Nakazono, Org. Lett. 2006, 8, 5133–5136;
- 13cJ. D. Crowley, K. D. Hänni, A.-L. Lee, D. A. Leigh, J. Am. Chem. Soc. 2007, 129, 12092–12093;
- 13dV. Aucagne, J. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, D. A. Leigh, P. J. Lusby, V. E. Ronaldson, A. M. Z. Slawin, A. Viterisi, D. B. Walker, J. Am. Chem. Soc. 2007, 129, 11950–11963;
- 13eJ. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, A.-L. Lee, D. A. Leigh, Angew. Chem. 2007, 119, 5811–5815;
10.1002/ange.200701678 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5709–5713;
- 13fS. M. Goldup, D. A. Leigh, P. J. Lusby, R. T. McBurney, A. M. Z. Slawin, Angew. Chem. 2008, 120, 3429–3432;
10.1002/ange.200705859 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3381–3384;
- 13gJ. Berná, S. M. Goldup, A.-L. Lee, D. A. Leigh, M. D. Symes, G. Teobaldi, F. Zerbetto, Angew. Chem. 2008, 120, 4464–4468;
10.1002/ange.200800891 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4392–4396;
- 13hY. Sato, R. Yamasaki, S. Saito, Angew. Chem. 2009, 121, 512–515; Angew. Chem. Int. Ed. 2009, 48, 504–507;
- 13iS. M. Goldup, D. A. Leigh, T. Long, P. R. McGonigal, M. D. Symes, J. Wu, J. Am. Chem. Soc. 2009, 131, 15924–15929;
- 13jJ. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh, R. T. McBurney, Chem. Soc. Rev. 2009, 38, 1530–1541;
- 13kS. M. Goldup, D. A. Leigh, P. R. McGonigal, V. E. Ronaldson, A. M. Z. Slawin, J. Am. Chem. Soc. 2010, 132, 315–320;
- 13lJ. D. Crowley, K. D. Hänni, D. A. Leigh, A. M. Z. Slawin, J. Am. Chem. Soc. 2010, 132, 5309–5314;
- 13mS. M. Goldup, D. A. Leigh, R. T. McBurney, P. R. McGonigal, A. Plant, Chem. Sci. 2010, 1, 383–386;
- 13nH. Lahlali, K. Jobe, M. Watkinson, S. M. Goldup, Angew. Chem. 2011, 123, 4237–4241;
10.1002/ange.201100415 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4151–4155;
- 13oH. M. Cheng, D. A. Leigh, F. Maffei, P. R. McGonigal, A. M. Z. Slawin, J. Wu, J. Am. Chem. Soc. 2011, 133, 12298–12303;
- 13pM. J. Langton, J. D. Matichak, A. L. Thompson, H. L. Anderson, Chem. Sci. 2011, 2, 1897–1901.
- 14
- 14aC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057–3062;
- 14bV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708–2711;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596–2599.10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 15K. D. Hänni, D. A. Leigh, Chem. Soc. Rev. 2010, 39, 1240–1251.
- 16Molecular modeling was carried using the SPARTAN package. SPARTAN ’06, 1.1, W. J. Hehre, Wavefunction, Inc., Irvine, CA, 2006.
- 17Upon adding [(CH3CN)4Cu]PF6 to a solution of 1 in chloroform a red, gummy solid formed instantaneously. The solid was insoluble in all common laboratory solvents and was thus assumed to be a result of rapid polymerization.
- 18The use of less than a stoichiometric amount of copper is to try to minimize the amount of CuI ions not coordinated to 1. “Free” CuI ions could catalyze the CuAAC reaction without the end groups passing through the ligand strand loop, thus generating unknot macrocycle 3. CuI ions can turn over during active-template CuAAC reactions [Ref 13 a,d,i,l] and therefore stoichiometric amounts of the metal are unnecessary.
- 19The assignment is confirmed as an AB system by a coupling constant value (JAB=13.2 Hz) that is unchanged when measured at 500 MHz (Figure 2 d) and 400 MHz (Figure 2 e), and 1H–1H COSY experiments that show that the two protons coupled in the signal are not coupled to other protons in the molecule.
- 20F. Vögtle, A. Hünten, E. Vogel, S. Buschbeck, O. Safarowsky, J. Recker, A.-H. Parham, M. Knott, W. M. Müller, U. Müller, Y. Okamoto, T. Kubota, W. Lindner, E. Francotte, S. Grimme, Angew. Chem. 2001, 113, 2534–2537;
10.1002/1521-3757(20010702)113:13<2534::AID-ANGE2534>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2468–2471.10.1002/1521-3773(20010702)40:13<2468::AID-ANIE2468>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 21B. J. McCullough, J. Kalapothakis, H. Eastwood, P. Kemper, D. MacMillan, K. Taylor, J. Dorin, P. E. Barran, Anal. Chem. 2008, 80, 6336–6344.
- 22D. Macmillan, M. De Cecco, N. L. Reynolds, L. F. A. Santos, P. E. Barran, J. R. Dorin, ChemBioChem 2011, DOI: .
- 23For all three isomers (1–3) the [M+2 H]2+ ion was the dominant charge state observed in the DT IM-MS experiments (see the Supporting Information). The absence of the [M+3 H]3+ species for the trefoil knot may be indicative of a compact conformation unable to support the electrostatic repulsions between three protons.
- 24E. R. Kay, D. A. Leigh, F. Zerbetto, Angew. Chem. 2007, 119, 72–196; Angew. Chem. Int. Ed. 2007, 46, 72–191.