Chiral Phosphoric Acid Catalyzed Desymmetrization of meso-1,3-Diones: Asymmetric Synthesis of Chiral Cyclohexenones†
Keiji Mori Dr.
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorTakuya Katoh
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorTohru Suzuki
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorTakuya Noji
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorMasahiro Yamanaka Prof. Dr.
Department of Chemistry, Rikkyo University, 3-34-1 Nishi-Ikebukuro, Toshima-ku, Tokyo 171-8501 (Japan)
Search for more papers by this authorTakahiko Akiyama Prof. Dr.
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorKeiji Mori Dr.
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorTakuya Katoh
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorTohru Suzuki
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorTakuya Noji
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorMasahiro Yamanaka Prof. Dr.
Department of Chemistry, Rikkyo University, 3-34-1 Nishi-Ikebukuro, Toshima-ku, Tokyo 171-8501 (Japan)
Search for more papers by this authorTakahiko Akiyama Prof. Dr.
Department of Chemistry, Faculty of Science, Gakushuin University, 1-5-1, Mejiro, Toshima-ku, Tokyo, 171-8588 (Japan), Fax: (+81) 3-5992-1029 http://www-cc.gakushuin.ac.jp/∼940020/akiyama_site/index_e.html
Search for more papers by this authorThis work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (Japan).
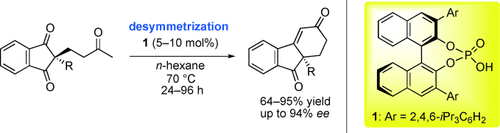
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200905271_sm_miscellaneous_information.pdf6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aU. Eder, G. Sauer, R. Wiechert, Angew. Chem. 1971, 83, 492–493;
10.1002/ange.19710831307 Google ScholarAngew. Chem. Int. Ed. Engl. 1971, 10, 496–497;
- 1bZ. G. Hajos, D. R. Parrish, J. Org. Chem. 1974, 39, 1615–1621;
- 1cR. A. Micheli, Z. G. Hajos, N. Cohen, D. R. Parrish, L. A. Poland, W. Sciamanna, M. A. Scott, P. A. Wehrli, J. Org. Chem. 1975, 40, 675–681;
- 1dZ. G. Hajos, D. R. Parrish, Org. Synth., Collect Vol. VII, Wiley, New York, 1990, pp. 363–369.
- 2
- 2aP. Wieland, K. Miescher, Helv. Chim. Acta 1950, 33, 2215–2228;
- 2bJ. Gutzwiller, A. Buchschacher, A. Furst, Synthesis 1977, 167–168;
- 2cS. Ramachandran, M. S. Newman, Org. Synth. 1961, 41, 38.
- 3For selected references, see:
- 3aP. Wieland, H. Unberwasser, G. Annre, K. Miescher, Helv. Chim. Acta 1953, 36, 1231–1241;
- 3bP. Wieland, G. Anner, K. Miescher, Helv. Chim. Acta 1953, 36, 1803–1809;
- 3cT. A. Spencer, T. D. Weaver, R. M. Villarica, R. J. Friary, J. Posler, M. A. Schwartz, J. Org. Chem. 1968, 33, 712–719;
- 3dS. J. Danishefsky, P. Cain, J. Am. Chem. Soc. 1975, 97, 5282–5284.
- 4For selected references, see:
- 4aE. J. Corey, M. Ohno, R. B. Mitra, P. A. Vatakencherry, J. Am. Chem. Soc. 1964, 86, 478–485;
- 4bE. J. Corey, M. Ohno, R. B. Mitra, R. A. Vatakencherry, J. Am. Chem. Soc. 1964, 86, 478–485;
- 4cJ. E. McMurry, S. J. Isser, J. Am. Chem. Soc. 1972, 94, 7132–7137;
- 4dR. C. A. Isaacs, R. M. J. Di Grandi, S. J. Danishefsky, J. Org. Chem. 1993, 58, 3938–3941;
- 4eL. A. Paquette, T.-Z. Wang, M. R. Sivik, J. Am. Chem. Soc. 1994, 116, 11323–11334;
- 4fT. J. Reddy, G. Bordeau, L. Trimble, Org. Lett. 2006, 8, 5585–5588;
- 4gS. Yamashita, K. Iso, M. Hirama, Org. Lett. 2008, 10, 3413–3415.
- 5
- 5aT. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. 2004, 116, 1592–1594;
10.1002/ange.200353240 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1566–1568;
- 5bD. Uraguchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 5356–5357.
- 6For reviews on Brønsted acid catalysis, see:
- 6aT. Akiyama, J. Itoh, K. Fuchibe, Adv. Synth. Catal. 2006, 348, 999–1010;
- 6bT. Akiyama, Chem. Rev. 2007, 107, 5744–5758;
- 6cS. J. Connon, Angew. Chem. 2006, 118, 4013–4016;
10.1002/ange.200600529 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3909–3912;
- 6dT. Akiyama in Acid Catalysis in Modern Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2008, pp. 62–107;
- 6eM. Terada, Chem. Commun. 2008, 4097–4112, and references therein.
- 7aE. B. Rowland, G. B. Rowland, E. Rivera-Otero, J. C. Antilla, J. Am. Chem. Soc. 2007, 129, 12084–12085;
- 7bD. K. Sala, A. Lattanzi, Org. Lett. 2009, 11, 3330–3333;
- 7cG. L. Hamilton, T. Kanai, F. D. Toste, J. Am. Chem. Soc. 2008, 130, 14984–14986.
- 8
- 8aS. Bahmanyar, K. N. Houk, J. Am. Chem. Soc. 2001, 123, 12911–12912;
- 8bL. Hoang, S. Bahmanyar, K. N. Houk, B. List, J. Am. Chem. Soc. 2003, 125, 16–17;
- 8cC. Allemann, R. Gordillo, F. R. Clemente, H.-Y. Cheong, K. N. Houk, Acc. Chem. Res. 2004, 37, 558–569.
- 9For our previous approach to chiral cyclohexenones, see: T. Akiyama, T. Katoh, K. Mori, Angew. Chem. 2009, 121, 4290–4292;
10.1002/ange.200901127 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4226–4228.
- 10The absolute configuration of 3 a was unambiguously established by single-crystal X-ray analysis of the corresponding bromide. CCDC 743645 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. See the Supporting Information.
- 11S. Takano, C. Kasahara, K. Ogasawara, J. Chem. Soc. Chem. Commun. 1981, 635–637.
- 12The absolute configuration of Hajos–Parrish and Wieland–Miescher ketones were determined by comparing optical rotation values with those reported. E. Lacoste, E. Vaique, M. Berlande, I. Pianet, J. M. Vincent, Y. Landais, Eur. J. Org. Chem. 2007, 167–177.
- 13Details of chemical models of ONIOM calculations are shown in the Supporting Information. All calculations were performed with the Gaussian 03 package, see: Gaussian 03, Revision E.01., M. J. Frisch, et al. Gaussian, Inc., Wallingford CT, 2004.
- 14For theoretical studies on chiral phosphoric acid catalysis, see:
- 14aM. Yamanaka, J. Itoh, K. Fuchibe, T. Akiyama, J. Am. Chem. Soc. 2007, 129, 6756–6764;
- 14bL. Simón, J. M. Goodman, J. Am. Chem. Soc. 2008, 130, 8741–8747;
- 14cT. Marcelli, P. Hammar, F. Himo, Chem. Eur. J. 2008, 14, 8562–8571;
- 14dL. Simón, J. M. Goodman, J. Am. Chem. Soc. 2009, 131, 4070–4077;
- 14eM. Yamanaka, T. Hirata, J. Org. Chem. 2009, 74, 3266–3271;
- 14fT. Akiyama, H. Morita, P. Bachu, K. Mori, M. Yamanaka, T. Hirata, Tetrahedron 2009, 65, 4950–4956.