Concise One-Pot Tandem Synthesis of Indoles and Isoquinolines from Amides†
Noriko Okamoto
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Department of Synthetic Organic Chemistry, Graduate School of Medical Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-Ku, Hiroshima 734-8553 (Japan)
Search for more papers by this authorYoshihisa Miwa Prof. Dr.
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Search for more papers by this authorHideki Minami
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Search for more papers by this authorKei Takeda Prof. Dr.
Department of Synthetic Organic Chemistry, Graduate School of Medical Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-Ku, Hiroshima 734-8553 (Japan)
Search for more papers by this authorReiko Yanada Prof. Dr.
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Search for more papers by this authorNoriko Okamoto
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Department of Synthetic Organic Chemistry, Graduate School of Medical Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-Ku, Hiroshima 734-8553 (Japan)
Search for more papers by this authorYoshihisa Miwa Prof. Dr.
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Search for more papers by this authorHideki Minami
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Search for more papers by this authorKei Takeda Prof. Dr.
Department of Synthetic Organic Chemistry, Graduate School of Medical Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-Ku, Hiroshima 734-8553 (Japan)
Search for more papers by this authorReiko Yanada Prof. Dr.
Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hirokoshingai, Kure , Hiroshima 737-0112 (Japan), Fax: (+81) 823-73-8981
Search for more papers by this authorThis work was supported by a Grant-in-Aid for Scientific Research(C) from JSPS KAKENHI (20590027).
Graphical Abstract
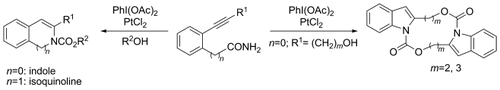
Heterocyclic hot pot: Platinum(II)-catalyzed syntheses of indoles and isoquinolines from isocyanates, which are derived from a Hofmann-type rearrangement of amides using a hypervalent iodine reagent, are described. C2-symmetric macrocyclic bis(indole)s can also be synthesized from transannulation of C2-symmetric macrocyclic bis(alkyne carbamate) intermediates.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200904960_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Indole synthesis:
- 1aN. Sakai, K. Annaka, A. Fujita, A. Sato, T. Konakahara, J. Org. Chem. 2008, 73, 4160–4165;
- 1bA. Fürstner, P. W. Davies, Angew. Chem. 2007, 119, 3478–3519;
10.1002/ange.200604335 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3410–3449;
- 1cY. Zhang, J. P. Donahue, C.-J. Li, Org. Lett. 2007, 9, 627–630;
- 1dG. R. Humphrey, J. T. Kuethe, Chem. Rev. 2006, 106, 2875–2911 and references therein;
- 1eF. Alonso, I. P. Beletskaya, M. Yus, Chem. Rev. 2004, 104, 3079–3159;
- 1fK. Hiroya, S. Itoh, T. Sakamoto, J. Org. Chem. 2004, 69, 1126–1136;
- 1gA. Takeda, S. Kamijo, Y. Yamamoto, J. Am. Chem. Soc. 2000, 122, 5662–5663.
- 2Isoquinoline synthesis:
- 2aD. Fischer, H. Tomeba, N. K. Pahadi, N. T. Patil, Z. Huo, Y. Yamamoto, J. Am. Chem. Soc. 2008, 130, 15720–15725;
- 2bT. Enomoto, S. Obika, Y. Yasui, Y. Takemoto, Synlett 2008, 1647–1650;
- 2cK. Gao, J. Wu, J. Org. Chem. 2007, 72, 8611–8613;
- 2dQ. Ding, Y. Ye, R. Fan, J. Wu, J. Org. Chem. 2007, 72, 5439–5442;
- 2eS. Su, J. A. Porco, Jr., Org. Lett. 2007, 9, 4983–4986;
- 2fQ. Ding, J. Wu, Org. Lett. 2007, 9, 4959–4962;
- 2gN. Asao, S. Yudha S., T. Nogami, Y. Yamamoto, Angew. Chem. 2005, 117, 5662–5664;
10.1002/ange.200500795 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5526–5528.
- 3
- 3aS. Obika, H. Kono, Y. Yasui, R. Yanada, Y. Takemoto, J. Org. Chem. 2007, 72, 4462–4468;
- 3bR. Yanada, S. Obika, H. Kono, Y. Takemoto, Angew. Chem. 2006, 118, 3906–3909;
10.1002/ange.200600408 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3822–3825.
- 4S. Kamijo, Y. Yamamoto, J. Org. Chem. 2003, 68, 4764–4771.
- 5Isocyanates prepared with hypervalent iodine reagents:
- 5aW. Liu, M. Buck, N. Chen, M. Shang, N. J. Taylor, J. Asoud, X. Wu, B. B. Hasinoff, G. I. Dmitrienko, Org. Lett. 2007, 9, 2915–2918;
- 5bR. M. Moriarty, C. J. Chany II, R. K. Vaid, O. Prakash, S. M. Tuladhar, J. Org. Chem. 1993, 58, 2478–2482.
- 6Isocyanates prepared with triphosgene:
- 6aS. Fustero, G. Chiva, J. Piera, J. F. Sanz-Cervera, A. Volonterio, M. Zanda, C. R. de Arellano, J. Org. Chem. 2009, 74, 3122–3132;
- 6bA. El Alaoui, F. Schmidt, C. Monneret, J.-C. Florent, J. Org. Chem. 2006, 71, 9628–9636;
- 6cT. Akiba, O. Tamura, S. Terashima, Org. Synth. 1998, 75, 45.
- 7The isocyanate was used without further purification because of its instability.
- 8
- 8aV. V. Zhdankin, P. J. Stang, Chem. Rev. 2002, 102, 2523–2584;
- 8bP. J. Stang, V. V. Zhdankin, Chem. Rev. 1996, 96, 1123–1178.
- 9CCDC 745937 (5 c); CCDC 745938 (7 a), and CCDC 745939 (8 a), contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 10
- 10aJ. Sivaguru, M. R. Solomon, T. Poon, S. Jockusch, S. G. Bosio, W. Adam, N. J. Turro, Acc. Chem. Res. 2008, 41, 387–400;
- 10bT. J. Harrison, B. O. Patrick, G. R. Dake, Org. Lett. 2007, 9, 367–370;
- 10cA. R. de Faria, E. L. Salvador, C. R. D. Correia, J. Org. Chem. 2002, 67, 3651–3661;
- 10dE. A. Severino, C. R. D. Correia, Org. Lett. 2000, 2, 3039–3042.
- 11
- 11aM. Sunkur, D. Baris, H. Hosgoren, M. Togrul, J. Org. Chem. 2008, 73, 2570–2575;
- 11bD. Demeter, P. Blanchard, M. Allain, I. Grosu, J. Roncali, J. Org. Chem. 2007, 72, 5285–5290;
- 11cP. Osswald, M. Reichert, G. Bringmann, F. Würthner, J. Org. Chem. 2007, 72, 3403–3411;
- 11dS. E. Gibson, N. Mainolfi, S. B. Kalindjian, P. T. Wright, A. J. P. White, Chem. Eur. J. 2005, 11, 69–80;
- 11eT. Fekner, J. Gallucci, M. K. Chan, J. Am. Chem. Soc. 2004, 126, 223–236;
- 11fW. S. Horne, C. D. Stout, M. R. Ghadiri, J. Am. Chem. Soc. 2003, 125, 9372–9376.
- 12
- 12aC. Han, S. Rangarajan, A. C. Voukides, A. B. Beeler, R. Johnson, J. A. Porco, Jr., Org. Lett. 2009, 11, 413–416;
- 12bS. Surprenant, W. D. Lubell, Org. Lett. 2006, 8, 2851–2854.
- 13
- 13aS. Avolio, K. Robertson, J. Ignacio, M. Hernando, J. DiMuzio, V. Summa, Bioorg. Med. Chem. Lett. 2009, 19, 2295–2298;
- 13bA. D. Abell, M. A. Jones, J. M. Coxon, J. D. Morton, S. G. Aitken, S. B. McNabb, H. Y.-Y. Lee, J. M. Mehrtens, N. A. Alexander, B. G. Stuart, A. T. Neffe, R. Bickerstaffe, Angew. Chem. 2009, 121, 1483–1486;
10.1002/ange.200805014 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1455–1458;
- 13cS. Venkatraman, F. Velazquez, W. Wu, M. Blackman, K. X. Chen, S. Bogen, L. Nair, X. Tong, R. Chase, A. Hart, S. Agrawal, J. Pichardo, A. Prongay, K.-C. Cheng, V. Girijavallabhan, J. Piwinski, N.-Y. Shih, F. G. Njoroge, J. Med. Chem. 2009, 52, 336–346;
- 13dG. Shen, M. Wang, T. R. Welch, B. S. J. Blagg, J. Org. Chem. 2006, 71, 7618–7631;
- 13eB. K. Albrecht, R. M. Williams, Org. Lett. 2003, 5, 197–200.
- 14 The yields of bis(indole)s 8 were better with the stepwise procedure than with the simultaneous procedure.
- 15 For example, trimeric indole was obtained as a minor product from 6 a. Details of the complex mixture are currently under investigation.