Two-Dimensional Triangular and Square Heterometallic Clusters: Influence of the Closed-Shell d10 Electronic Configuration†
Sabrina Sculfort
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorPierre Croizat
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorAbdelatif Messaoudi
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorMarc Bénard Dr.
Laboratoire de Chimie Quantique, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France)
Search for more papers by this authorMarie-Madeleine Rohmer Dr.
Laboratoire de Chimie Quantique, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France)
Search for more papers by this authorRichard Welter Prof.
Laboratoire DECOMET, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France)
Search for more papers by this authorPierre Braunstein Dr.
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorSabrina Sculfort
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorPierre Croizat
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorAbdelatif Messaoudi
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorMarc Bénard Dr.
Laboratoire de Chimie Quantique, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France)
Search for more papers by this authorMarie-Madeleine Rohmer Dr.
Laboratoire de Chimie Quantique, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France)
Search for more papers by this authorRichard Welter Prof.
Laboratoire DECOMET, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France)
Search for more papers by this authorPierre Braunstein Dr.
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg (France), Fax: (+33) 3-6885-1322
Search for more papers by this authorSupport from the CNRS, the Ministère de l’Enseignement Supérieur et de la Recherche, the DFH/UFA, the DFG International Research Training Group GRK532, the IDRIS and CINES computer centers, and the Agence Nationale de la Recherche (ANR-06-BLAN-410) is gratefully acknowledged.
Graphical Abstract
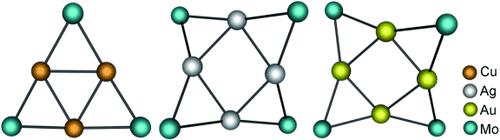
Gilded rafts: Oligomeric 2D raft clusters {M[m]}n (M=Cu, n=3; M=Ag or Au, n=4; see picture) with the same bridging metalloligand [m]={CpMo(CO)3} were prepared and structurally characterized. The ν2-triangular (M=Cu) or ν2-square (M=Ag, Au) structures of their metal–metal-bonded cores allow comparative evaluation of the d10⋅⋅⋅d10 interactions, and theoretical calculations point to a favorable contribution of diagonal Au⋅⋅⋅Au or Ag⋅⋅⋅Ag interactions.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200903895_sm_miscellaneous_information.pdf2.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1See for example,
- 1a Metal Clusters in Chemistry (Eds.: ), Wiley-VCH, Weinheim, 1999;
10.1002/9783527618316 Google Scholar
- 1b Catalysis by Di- and Polynuclear Metal Cluster Complexes (Eds.: ), Wiley-VCH, New York, 1998;
- 1c The Chemistry of Metal Cluster Complexes (Eds.: ), VCH, New York, 1990.
- 2See, for example, G. Schmid, Chem. Soc. Rev. 2008, 37, 1909–1930, and the articles in this special issue dedicated to “Gold, Chemistry, Materials and Catalysis”.
- 3G. J. Hutchings, Dalton Trans. 2008, 5523, and references therein.
- 4C. E. Coffey, J. Lewis, R. S. Nyholm, J. Chem. Soc. 1964, 1741–1749.
- 5
- 5aI. D. Salter in Comprehensive Organometallic Chemistry II, Vol. 10 (Eds.: ), Pergamon, New York, 1995, p. 255;
10.1016/B978-008046519-7.00089-7 Google Scholar
- 5bM. J. Chetcuti in Comprehensive Organometallic Chemistry II, Vol. 10 (Eds.: ), Pergamon, New York, 1995, p. 23;
10.1016/B978-008046519-7.00086-1 Google Scholar
- 5cV. Ritleng, M. J. Chetcuti, Chem. Rev. 2007, 107, 797–858, and references therein.
- 6
- 6aP. Pyykkö, Chem. Rev. 1997, 97, 597–636;
- 6bP. Pyykkö, Angew. Chem. 2002, 114, 3723–3728;
10.1002/1521-3757(20021004)114:19<3723::AID-ANGE3723>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3573–3578;10.1002/1521-3773(20021004)41:19<3573::AID-ANIE3573>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 6cP. Pyykkö, Angew. Chem. 2004, 116, 4512–4557;
10.1002/ange.200300624 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4412–4456;
- 6dM. A. Carvajal, S. Alvarez, J. J. Novoa, Chem. Eur. J. 2004, 10, 2117–2132;
- 6eP. Pyykkö, Inorg. Chim. Acta 2005, 358, 4113–4130;
- 6fP. Pyykkö, Chem. Soc. Rev. 2008, 37, 1967–1997.
- 7M. Bénard, U. Bodensieck, P. Braunstein, M. Knorr, M. Strampfer, C. Strohmann, Angew. Chem. 1997, 109, 2890–2893;
10.1002/ange.19971092420 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2758–2761; W. Schuh, P. Braunstein, M. Bénard, M.-M. Rohmer, R. Welter, Angew. Chem. 2003, 115, 2211–2214;10.1002/ange.200250503 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 2161–2164; W. Schuh, P. Braunstein, M. Bénard, M.-M. Rohmer, R. Welter, J. Am. Chem. Soc. 2005, 127, 10250–10258.
- 8CCDC 720822, 720823, 720824, 720825, 720826 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9aA. F. Holleman, E. Wiberg, Lehrbuch der Anorganischen Chemie, 91st ed., W. de Gruyter, Berlin, 1985;
10.1515/9783110838176 Google Scholar
- 9bB. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverria, E. Cremades, F. Rarragán, S. Alvarez, Dalton Trans. 2008, 2832–2838;
- 9cA. Bondi, J. Phys. Chem. 1964, 68, 441–451.
- 10All calculations were carried out with the Gaussian 03 software suite.[11] Stuttgart–Dresden relativistic electron core potentials were used for all metals. The valence shell of the metal atoms, namely, the nspd and (n+1)s shells, were described by means of triple-ζ Gaussian basis sets, supplemented with diffuse p functions and with one f-type polarization function (G. Frenking et al., Chem. Phys. Lett. 1993, 208, 111–114). In the DFT/B3LYP calculations, C, O, and H atoms were described by all-electron 6-311G** basis sets. The single-point MP2 calculations were carried out on the geometry optimized at the DFT level. The same basis sets were used for the metal atoms, but the other atoms were described with smaller, LANL2DZ basis sets.
- 11Gaussian 03, Revision B.05, M. J. Frisch et al., Gaussian, Inc., Pittsburgh, PA, 2003.
- 12In the triangular clusters with C1 symmetry, 11 out of 12 carbonyl ligands display a semibridging bonding mode. At variance with the case of the Cs triangles, this coordination mode is retained for all three coinage metals, and thus gives rise to a large average deformation energy for the {MoCp(CO)3} fragments (Figure 5). All computed structures are displayed in the Supporting Information.
- 13A coordination mode in which all carbonyl ligands are engaged in semibridging bonds with copper was designed in square-like model complexes of Cu with D2 and S4 symmetries. The 12 semibridging interactions subsisted after geometry optimization, and the total bond energies differed by less than 1 kJ mol−1 from that of the most stable square structure with S4 symmetry and nine semibridging interactions. Similar configurations optimized with Ag and Au are however significantly destabilized.
- 14P. Pyykkö, J. Li, N. Runeberg, Chem. Phys. Lett. 1994, 218, 133–138.
- 15G. Doyle, K. A. Eriksen, D. Van Engen, J. Am. Chem. Soc. 1986, 108, 445–451.
- 16T. H. Lemmen, J. C. Huffman, K. G. Caulton, Angew. Chem. 1986, 98, 267–268; Angew. Chem. Int. Ed. Engl. 1986, 25, 262–264.
- 17
- 17aP. Klüfers, Angew. Chem. 1984, 96, 288–290; Angew. Chem. Int. Ed. Engl. 1984, 23, 307–308;
- 17bP. Klüfers, Z. Kristallogr. 1984, 166, 143–151.
- 18
- 18aV. G. Albano, F. Azzaroni, M. C. Iapalucci, G. Longoni, M. Monari, S. Mulley, D. M. Proserpio, A. Sironi, Inorg. Chem. 1994, 33, 5320–5328;
- 18bV. G. Albano, F. Calderoni, M. C. Iapalucci, G. Longoni, M. Monari, J. Chem. Soc. Chem. Commun. 1995, 433–434.