“On-Off” Multivalent Recognition: Degradable Dendrons for Temporary High-Affinity DNA Binding†
Daniel J. Welsh
Department of Chemistry, University of York, Heslington, York, YO10 5DD (UK), Fax: (+44) 1904-432-516, http://www.york.ac.uk/depts/chem/staff/dks.html
Search for more papers by this authorSimon P. Jones Dr.
Department of Chemistry, University of York, Heslington, York, YO10 5DD (UK), Fax: (+44) 1904-432-516, http://www.york.ac.uk/depts/chem/staff/dks.html
Search for more papers by this authorDavid K. Smith Prof.
Department of Chemistry, University of York, Heslington, York, YO10 5DD (UK), Fax: (+44) 1904-432-516, http://www.york.ac.uk/depts/chem/staff/dks.html
Search for more papers by this authorDaniel J. Welsh
Department of Chemistry, University of York, Heslington, York, YO10 5DD (UK), Fax: (+44) 1904-432-516, http://www.york.ac.uk/depts/chem/staff/dks.html
Search for more papers by this authorSimon P. Jones Dr.
Department of Chemistry, University of York, Heslington, York, YO10 5DD (UK), Fax: (+44) 1904-432-516, http://www.york.ac.uk/depts/chem/staff/dks.html
Search for more papers by this authorDavid K. Smith Prof.
Department of Chemistry, University of York, Heslington, York, YO10 5DD (UK), Fax: (+44) 1904-432-516, http://www.york.ac.uk/depts/chem/staff/dks.html
Search for more papers by this authorWe acknowledge financial support from EPSRC (EP/C534395/1).
Graphical Abstract
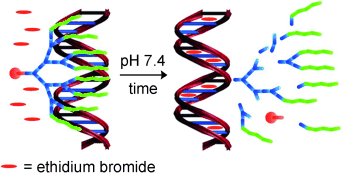
Now you bind it—now you don't! Chemical degradation of a dendritic scaffold allows multivalent interactions with DNA to be “switched off” as the multivalent array of ligands breaks down into smaller fragments, offering an approach by which a molecule can be temporarily endowed with high affinity for a biological target—an important concept in the development of new synthetic systems to intervene in biological pathways.
Abstract
Now you bind it—now you don't! Chemical degradation of a dendritic scaffold allows multivalent interactions with DNA to be “switched off” as the multivalent array of ligands breaks down into smaller fragments, offering an approach by which a molecule can be temporarily endowed with high affinity for a biological target—an important concept in the development of new synthetic systems to intervene in biological pathways.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200900401_sm_miscellaneous_information.pdf366 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Mammen, S.-K. Choi, G. M. Whitesides, Angew. Chem. 1998, 110, 2908–2953;
10.1002/(SICI)1521-3757(19981016)110:20<2908::AID-ANGE2908>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2754–2794;10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 1bA. Mulder, J. Huskens, D. N. Reinhoudt, Org. Biomol. Chem. 2004, 2, 3409–3424;
- 1cJ. D. Badjic, A. Nelson, S. J. Cantrill, W. B. Turnbull, J. F. Stoddart, Acc. Chem. Res. 2005, 38, 723–732.
- 2J. Huskens, A. Mulder, T. Auletta, C. A. Nijhuis, M. J. W. Ludden, D. N. Reinhoudt, J. Am. Chem. Soc. 2004, 126, 6784–6797.
- 3
- 3aC. C. Lee, J. A. MacKay, J. M. J. Fréchet, F. C. Szoka, Nat. Biotechnol. 2005, 23, 1517–1526;
- 3bU. Boas, J. B. Christensen, P. M. H. Heegard, Dendrimers in Medicine and Biotechnology, RSC, Cambridge, 2006;
- 3cS. Svenson, D. A. Tomalia, Adv. Drug Delivery Rev. 2005, 57, 2106–2129;
- 3dD. K. Smith, Curr. Top. Med. Chem. 2008, 8, 1187–1203.
- 4
- 4aY. M. Chabre, R. Roy, Curr. Top. Med. Chem. 2008, 8, 1237–1285;
- 4bR. J. Pieters, Med. Res. Rev. 2007, 27, 796–816.
- 5M. A. Kostiainen, J. G. Hardy, D. K. Smith, Angew. Chem. 2005, 117, 2612–2615;
10.1002/ange.200500066 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2556–2559.
- 6
- 6aC. W. Tabor, H. Tabor, Annu. Rev. Biochem. 1984, 740–790;
- 6bV. Vijayanathan, T. Thomas, A. Shirahata, T. J. Thomas, Biochemistry 2001, 40, 13644–13651;
- 6cK. Igarashi, K. Kashiwagi, Biochem. Biophys. Res. Commun. 2000, 271, 559–564.
- 7
- 7aS. P. Jones, N. P. Gabrielson, D. W. Pack, D. K. Smith, Chem. Commun. 2008, 4700–4702;
- 7bJ. G. Hardy, M. A. Kostiainen, D. K. Smith, N. P. Gabrielson, D. W. Pack, Bioconjugate Chem. 2006, 17, 172–178;
- 7cJ. G. Hardy, C. S. Love, N. P. Gabrielson, D. W. Pack, D. K. Smith, Org. Biomol. Chem. 2009, 7, 789–793.
- 8
- 8aM. A. Kostiainen, G. R. Szilvay, D. K. Smith, M. B. Linder, O. Ikkala, Angew. Chem. 2006, 118, 3618–3622;
10.1002/ange.200504540 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3538–3542;
- 8bM. A. Kostiainen, G. R. Szilvay, J. Lehtinen, D. K. Smith, M. B. Linder, A. Urtti, O. Ikkala, ACS Nano 2007, 1, 103–113.
- 9J.-H. S. Kuo, Y.-L. Lin, J. Biotechnol. 2007, 129, 383–390.
- 10For examples see:
- 10aJ. Luten, C. F. van Nostruin, S. C. De Smedt, W. E. Hennink, J. Controlled Release 2008, 126, 97–110;
- 10bJ. A. Wolff, D. B. Rozema, Mol. Ther. 2008, 16, 8–15;
- 10cB. Martin, M. Sainlos, A. Aissaoui, N. Oudrhiri, M. Hauchecorne, J.-P. Vigneron, J.-M. Lehn, P. Lehn, Curr. Pharm. Des. 2005, 11, 375–394;
- 10dA. Roosjen, J. Smisterova, C. Driessen, J. T. Anders, A. Wagenaar, D. Hoekstra, R. Hulst, J. B. F. N. Engberts, Eur. J. Org. Chem. 2002, 1271–1277;
- 10eJ. B. Wong, S. Grosse, A. B. Tabor, S. L. Hart, H. C. Hailes, Mol. Biosyst. 2008, 4, 532–541;
- 10fH. G. Chen, H. Z. Zhang, C. M. McCallum, F. C. Szoka, X. Guo, J. Med. Chem. 2007, 50, 4269–4278.
- 11
- 11aR. J. Amir, N. Pessah, M. Shamis, D. Shabat, Angew. Chem. 2003, 115, 4632–4637;
10.1002/ange.200351962 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4494–4499;
- 11bM. Shamis, H. N. Lode, D. Shabat, J. Am. Chem. Soc. 2004, 126, 1726–1731;
- 11cR. Perry, R. J. Amir, D. Shabat, New J. Chem. 2007, 31, 1307–1312;
- 11dA. Sagi, E. Segal, R. Satchi-Fainaro, D. Shabat, Bioorg. Med. Chem. 2007, 15, 3720–3727;
- 11eE. Sella, D. Shabat, Chem. Commun. 2008, 5701–5703.
- 12
- 12aM. L. Szalai, R. M. Kevwitch, D. V. McGrath, J. Am. Chem. Soc. 2003, 125, 15688–15689;
- 12bR. M. Kevwitch, D. V. McGrath, New J. Chem. 2007, 31, 1332–1336.
- 13F. M. H. de Groot, C. Albrecht, R. Koekkoek, P. H. Beusker, H. W. Scheeren, Angew. Chem. 2003, 115, 4628–4632;
10.1002/ange.200351942 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4490–4494.
- 14
- 14aE. R. Gillies, E. Dy, J. M. J. Fréchet, F. C. Szoka, Mol. Pharm. 2005, 2, 129–138;
- 14bA. Almutairi, S. J. Guillaudeu, M. Y. Berezin, S. Achilefu, J. M. J. Fréchet, J. Am. Chem. Soc. 2008, 130, 444–445;
- 14cA. Almutairi, W. J. Akers, M. Y. Berezin, S. Achilefu, J. M. J. Fréchet, Mol. Pharm. 2008, 5, 1103–1110.
- 15M. A. Kostiainen, D. K. Smith, O. Ikkala, Angew. Chem. 2007, 119, 7744–7748;
10.1002/ange.200701200 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7600–7604.
- 16
- 16aH. Ihre, A. Hult, E. Soederlind, J. Am. Chem. Soc. 1996, 118, 6388–6395;
- 16bH. Ihre, A. Hult, J. M. J. Fréchet, I. Gitsov, Macromolecules 1998, 31, 4061–4068;
- 16cH. Ihre, O. L. Padilla de Jesus, J. M. J. Fréchet, J. Am. Chem. Soc. 2001, 123, 5908–5917;
- 16dE. R. Gillies, J. M. J. Fréchet, J. Am. Chem. Soc. 2002, 124, 14137–14146;
- 16eC. C. Lee, E. R. Gillies, M. E. Fox, S. J. Guillaudeu, J. M. J. Fréchet, E. E. Dy, F. C. Szoka, Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654.
- 17
- 17aB. F. Cain, B. C. Baguley, W. A. Denny, J. Med. Chem. 1978, 21, 658–668;
- 17bH. Gershon, R. Ghirlando, S. B. Guttman, A. Minsky, Biochemistry 1993, 32, 7143–7151;
- 17cR. Zadmard, T. Schrader, Angew. Chem. 2006, 118, 2769–2772;
10.1002/ange.200502946 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2703–2706.
- 18
- 18aG. R. Newkome, X. Lin, Macromolecules 1991, 24, 1443–1444;
- 18bJ. K. Young, G. R. Baker, G. R. Newkome, K. F. Morris, C. S. Johnson, Jr., Macromolecules 1994, 27, 3464–3471.
- 19In their own right, these dendrons (e.g. G2-amide) are unable to effectively transfect cells, primarily owing to inefficient escape from endosomal vesicles. However, we recently reported (Ref. [7a]) that modification of the dendron focal point of G2-amide with hydrophobic groups can significantly enhance the gene delivery profile. We are therefore currently in the process of synthetically modifying these new degradable dendrons in an analogous manner, so that their gene delivery characteristics can be fully investigated.