Platinum(II)-Catalyzed Intramolecular Cyclization of o-Substituted Aryl Alkynes through sp3 CH Activation†
Shangdong Yang
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorZigang Li
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorXing Jian
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorChuan He Prof.
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorShangdong Yang
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorZigang Li
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorXing Jian
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorChuan He Prof.
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA), Fax: (+1) 773-702-0805, http://he-group.uchicago.edu/
Search for more papers by this authorThis work was supported by the University of Chicago. We thank Dr. J. Halpern for the generous gift of Pt salts.
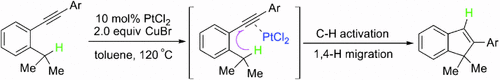
Graphical Abstract
Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200900368_sm_miscellaneous_information.pdf6.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. A. Arndtsen, R. G. Bergman, T. A. Mobley, T. H. Peterson, Acc. Chem. Res. 1995, 28, 154;
- 1bS. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani, Nature 1993, 366, 529;
- 1cG. Dyker, Angew. Chem. 1999, 111, 1808;
10.1002/(SICI)1521-3757(19990614)111:12<1808::AID-ANGE1808>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1698;10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 1dH. M. L. Davies, E. G. Antoulinakis, J. Organomet. Chem. 2001, 617–618, 47;
- 1eC. Jia, T. Kitamura, Y. Fujiwara, Acc. Chem. Res. 2001, 34, 633;
- 1fJ. A. Labinger, J. E. Bercaw, Nature 2002, 417, 507;
- 1gF. Kakiuchi, S. Murai, Acc. Chem. Res. 2002, 35, 826;
- 1hV. Ritleng, C. Sirlin, M. Pfeffer, Chem. Rev. 2002, 102, 1731;
- 1iF. Kakiuchi, N. Chatani, Adv. Synth. Catal. 2003, 345, 1077;
- 1jK. Godula, D. Sames, Science 2006, 312, 67;
- 1kO. Daugulis, V. G. Zaitsev, D. Shabashov, Q. N. Pham, A. Lazareva, Synlett 2006, 3382;
- 1lZ. Li, D. S. Bohle, C.-J. Li, Proc. Natl. Acad. Sci. USA 2006, 103, 8928;
- 1mH.-Y. Thu, W.-Y. Yu, C.-M. Che, J. Am. Chem. Soc. 2006, 128, 9048;
- 1nA. R. Dick, M. S. Sanford, Tetrahedron 2006, 62, 2439;
- 1oJ.-Q. Yu, R. Giri, X. Chen, Org. Biomol. Chem. 2006, 4, 4041;
- 1pZ. Shi, B. Li, S. Yang, Synlett 2008, 949.
- 2
- 2aN. F. Goldshleger, V. V. Eskova, A. E. Shilov, A. A. Shteinman, Zh. Fiz. Khim. 1972, 46, 1353 (Engl. trans. 1972, 46, 785–786);
- 2bT. J. Williams, A. J. M. Caffyn, N. Hazari, P. F. Oblad, J. A. Labinger, J. E. Bercaw, J. Am. Chem. Soc. 2008, 130, 2418;
- 2cG. Chen, J. A. Labinger, J. E. Bercaw, Proc. Natl. Acad. Sci. USA 2007, 104, 6915;
- 2dV. R. Ziatdinov, J. Oxgaard, O. A. Mironov, K. J. H. Young, W. A. Goddard III, R. A. Periana, J. Am. Chem. Soc. 2006, 128, 7404;
- 2eM. P. Jensen, D. D. Wick, S. Reinartz, P. S. White, J. L. Templeton, K. I. Goldberg, J. Am. Chem. Soc. 2003, 125, 8614;
- 2fJ. C. Thomas, J. C. Peters, J. Am. Chem. Soc. 2003, 125, 8870.
- 3
- 3aA. E. Shilov, G. B. Shulpin, Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes, Kluwer, Boston, 2000;
- 3bA. R. Chianese, S. J. Lee, M. R. Gagné, Angew. Chem. 2007, 119, 4118;
10.1002/ange.200603954 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4042;
- 3cN. A. Williams, Y. Uchimaru, M. Tanaka, J. Chem. Soc. Chem. Commun. 1995, 1129;
- 3dH. A. Zhong, J. A. Labinger, J. E. Bercaw, J. Am. Chem. Soc. 2002, 124, 1378;
- 3eW. V. Konze, B. L. Scott, G. J. Kubas, J. Am. Chem. Soc. 2002, 124, 12550;
- 3fS. M. Kloek, K. I. Goldberg, J. Am. Chem. Soc. 2007, 129, 3460.
- 4
- 4aI. Nakamura, Y. Yamamoto, Chem. Rev. 2004, 104, 2127;
- 4bI. Nakamura, G. B. Bajracharya, H. Wu, K. Oishi, Y. Mizushima, I. D. Gridnev, Y. Yamamoto, J. Am. Chem. Soc. 2004, 126, 15423;
- 4cI. Nakamura, Y. Mizushima, Y. Yamamoto, J. Am. Chem. Soc. 2005, 127, 15022;
- 4dC. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura Y. Fujiwara, Science 2000, 287, 1992;
- 4eA. F. Fürstner, P. W. Davies, Angew. Chem. 2007, 119, 3478;
10.1002/ange.200604335 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3410;
- 4fL. Añorbe, G. Domínguez, J. Pérez-Castells, Chem. Eur. J. 2004, 10, 4938;
- 4gB. Martín-Matute, C. Nevado, D. J. Cárdenas, A. M. Echavarren, J. Am. Chem. Soc. 2003, 125, 5757.
- 5
- 5aL. C. Kao, A. Sen, J. Chem. Soc. Chem. Commun. 1991, 1242;
- 5bB. D. Dangel, J. A. Johnson, D. Sames, J. Am. Chem. Soc. 2001, 123, 8149;
- 5cJ. M. Lee, S. Chang, Tetrahedron Lett. 2006, 47, 1375.
- 6
- 6aU. Akbulut, A. Khurshid, B. Hacioglu, L. Toppare, Polymer 1990, 31, 1343;
- 6bJ. Barberá, O. A. Rakitin, M. B. Ros, T. Torroba, Angew. Chem. 1998, 110, 308;
10.1002/(SICI)1521-3757(19980202)110:3<308::AID-ANGE308>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1998, 37, 296;10.1002/(SICI)1521-3773(19980216)37:3<296::AID-ANIE296>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 6cC. Bolm, A. Korte, J. Legros, Synlett 2004, 13, 2397;
- 6dV. Cadierno, J. Diez, G. M. Pilar, J. Gimeno, E. Lastra, Coord. Chem. Rev. 1999, 193, 147;
- 6eD. Zargarian, Coord. Chem. Rev. 2002, 233, 157;
- 6fR. Leino, P. Lehmus, A. Lehtonen, Eur. J. Inorg. Chem. 2004, 3201.
- 7G. B. Bajracharya, N. K. Pahadi, I. D. Gridnev, Y. Yamamoto, J. Org. Chem. 2006, 71, 6204.
- 8Z. Li, D. A. Capretto, R. O. Rahaman, C. He, J. Am. Chem. Soc. 2007, 129, 12058.
- 9
- 9aC.-H. Oh, J. H. Lee, S. J. Lee, J. I. Kim, C. S. Hong, Angew. Chem. 2008, 120, 7615;
10.1002/ange.200802425 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7505;
- 9bH. Funami, H. Kusama, N. Iwasawa, Angew. Chem. 2007, 119, 927;
10.1002/ange.200603986 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 909;
- 9cB. P. Taduri, Y.-F. Ran, C.-W. Huang, R.-S. Liu, Org. Lett. 2006, 8, 883;
- 9dD. Hildebrandt, W. Hüggenberg, M. Kanthak, T. Plöger, I. M. Müller, G. Dyker, Chem. Commun. 2006, 2260;
- 9eH. O. Chang, V. R. Reddy, A. Kim, C. Y. Rhim, Tetrahedron Lett. 2006, 47, 5307;
- 9fA. Fürstner, P. W. Davies, J. Am. Chem. Soc. 2005, 127, 15024;
- 9gB. Martín-Matute, D. J. Cárdenas, A. M. Echavarren, Angew. Chem. 2001, 113, 4890;
Angew. Chem. Int. Ed. 2001, 40, 4754;
10.1002/1521-3773(20011217)40:24<4754::AID-ANIE4754>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 9hL. Zhang, J. Sun, S. A. Kozmin, Adv. Synth. Catal. 2006, 348, 2271.
- 10
- 10aJ. Tsuji, Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis, Wiley, New York, 2002;
10.1002/0470854766 Google Scholar
- 10bY. Harada, J. Nakanishi, H. Fujihara, M. Tobisu, Y. Fukumoto, N. Chatani, J. Am. Chem. Soc. 2007, 129, 5766;
- 10cA. Odedra, S. Datta, R.-S. Liu, J. Org. Chem. 2007, 72, 3289;
- 10dH. Tsukamoto, T. Ueno, Y. Kondo, Org. Lett. 2007, 9, 3033;
- 10eI. Nakamura, Y. Mizushima, I. D. Gridnev, Y. Yamamoto, J. Am. Chem. Soc. 2005, 127, 9844;
- 10fP. Dubé, F. D. Toste, J. Am. Chem. Soc. 2006, 128, 12062;
- 10gI. Ojima, A. C. Moralee, V. C. Vassar, Top. Catal. 2002, 19, 89;
- 10hJ. M. Joo, Y. Yuan, C. Lee, J. Am. Chem. Soc. 2006, 128, 14818.
- 11
- 11aJ. Almy, D. J. Cram, J. Am. Chem. Soc. 1970, 92, 4316;
- 11bJ. A. Berson, G. B. Aspelin, Tetrahedron 1964, 20, 2697.
- 12
- 12aM A. Campo, Q. Huang, T. Yao, Q. Tian, R. C. Larock, J. Am. Chem. Soc. 2003, 125, 11506;
- 12bL. Peng, L. T. Scott, J. Am. Chem. Soc. 2005, 127, 16518;
- 12cA. Singh, P. R. Sharp, J. Am. Chem. Soc. 2006, 128, 5998.