Sequential Asymmetric Polyketide Heterocyclization Catalyzed by a Single Cytochrome P450 Monooxygenase (AurH)†
Martin E. A. Richter
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Search for more papers by this authorNelly Traitcheva
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Search for more papers by this authorUwe Knüpfer
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Search for more papers by this authorChristian Hertweck Prof. Dr.
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Friedrich Schiller University, Jena (Germany)
Search for more papers by this authorMartin E. A. Richter
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Search for more papers by this authorNelly Traitcheva
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Search for more papers by this authorUwe Knüpfer
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Search for more papers by this authorChristian Hertweck Prof. Dr.
Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstrasse 11a, 07745 Jena (Germany), Fax: (+49) 3641-532-0804
Friedrich Schiller University, Jena (Germany)
Search for more papers by this authorThis research was supported financially by the DFG within the framework of the SPP 1152 (EvoMet). We thank F. Rhein for NMR spectroscopic measurements.
Graphical Abstract
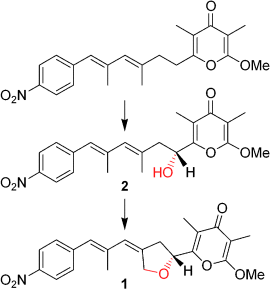
Two in one: In vitro biotransformations with the cytochrome P450 monooxygenase AurH from the aureothin (1) biosynthetic pathway provided direct experimental evidence that a single monooxygenase can install two CO bonds sequentially to form a tetrahydrofuran ring. Structural elucidation of the intermediate 2 revealed the order of bond formation and the stereochemical course of this unprecedented oxygenation–heterocyclization reaction.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200803714_sm_miscellaneous_information.pdf694.1 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. O'Hagan, The Polyketide Metabolites, Ellis Horwood, Chichester, 1991.
- 2H. B. Bode, A. Zeeck, J. Chem. Soc. Perkin Trans. 1 2000, 2665–2670.
- 3A. J. Woo, W. R. Strohl, N. D. Priestley, Antimicrob. Agents Chemother. 1999, 43, 1662–1668.
- 4T. Weber, K. J. Laiple, E. K. Pross, A. Textor, S. Grond, K. Welzel, S. Pelzer, A. Vente, W. Wohlleben, Chem. Biol. 2008, 15, 175–188.
- 5M. Oliynyk, C. B. Stark, A. Bhatt, M. A. Jones, Z. A. Hughes-Thomas, C. Wilkinson, Z. Oliynyk, Y. Demydchuk, J. Staunton, P. F. Leadlay, Mol. Microbiol. 2003, 49, 1179–1190.
- 6B. M. Harvey, T. Mironenko, Y. Sun, H. Hong, Z. Deng, P. F. Leadlay, K. J. Weissman, S. F. Haydock, Chem. Biol. 2007, 14, 703–714.
- 7Y. Sun, X. Zhou, H. Dong, G. Tu, M. Wang, B. Wang, Z. Deng, Chem. Biol. 2003, 10, 431–441.
- 8Y. Demydchuk, Y. Sun, H. Hong, J. Staunton, J. B. Spencer, P. F. Leadlay, ChemBioChem 2008, 9, 1136–1145.
- 9A. Bhatt, C. B. W. Stark, B. M. Harvey, A. R. Gallimore, Y. A. Demydchuk, J. B. Spencer, J. Staunton, P. F. Leadlay, Angew. Chem. 2005, 117, 7237–7240;
10.1002/ange.200501757 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7075–7078.
- 10A. R. Gallimore, C. B. Stark, A. Bhatt, B. M. Harvey, Y. Demydchuck, V. Bolanos-Garcia, D. J. Fowler, J. Staunton, P. F. Leadlay, J. B. Spencer, Chem. Biol. 2006, 13, 453–460.
- 11A. R. Gallimore, J. B. Spencer, Angew. Chem. 2006, 118, 4514–4521;
10.1002/ange.200504284 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4406–4413.
- 12K. M. Henry, C. A. Townsend, J. Am. Chem. Soc. 2005, 127, 3724–3733.
- 13J. He, C. Hertweck, J. Am. Chem. Soc. 2004, 126, 3694–3695.
- 14J. He, C. Hertweck, Chem. Biol. 2003, 10, 1225–1232.
- 15J. He, C. Hertweck, ChemBioChem 2005, 6, 908–912.
- 16N. Traitcheva, H. Jenke-Kodama, J. He, E. Dittmann, C. Hertweck, ChemBioChem 2007, 8, 1841–1849.
- 17M. Müller, J. He, C. Hertweck, ChemBioChem 2006, 7, 37–39.
- 18J. He, M. Müller, C. Hertweck, J. Am. Chem. Soc. 2004, 126, 16742–16743.
- 19R. Winkler, M. Richter, U. Knüpfer, D. Merten, C. Hertweck, Angew. Chem. 2006, 118, 8184–8186; Angew. Chem. Int. Ed. 2006, 45, 8016–8018.
- 20Z. Xu, A. Magyar, C. Hertweck, J. Am. Chem. Soc. 2007, 129, 6022–6030.
- 21Y. Ishibashi, S. Ohba, S. Nishiyama, S. Yamamura, Bull. Chem. Soc. Jpn. 1995, 68, 3643–3649.
- 22I. Ohtani, T. Kusumi, Y. Kashman, H. Kakisawa, J. Am. Chem. Soc. 1991, 113, 4092–4096.
- 23Y. Ishibashi, S. Ohaba, S. Nishiyama, S. Yamamura, Bull. Chem. Soc. Jpn. 1995, 68, 3643–3649.
- 24E. M. Isin, F. P. Guengerich, Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 314–329.
- 25D. Werck-Reichhart, R. Feyereisen, Genome Biol. 2000, 1, 3003.
10.1186/gb-2000-1-6-reviews3003 Google Scholar
- 26L. L. Wong, Curr. Opin. Chem. Biol. 1998, 2, 263–268.
- 27M. C. Rojas, P. Hedden, P. Gaskin, B. Tudzynski, Proc. Natl. Acad. Sci. USA 2001, 98, 5838–5843.
- 28W. Nam, Acc. Chem. Res. 2007, 40, 522–531.
- 29M. Newcomb, P. F. Hollenberg, M. J. Coon, Arch. Biochem. Biophys. 2003, 409, 72–79.
- 30T. Hashimoto, J. Matsuda, Y. Yamada, FEBS Lett. 1993, 329, 35–39.