Sequential Norrish Type II Photoelimination and Intramolecular Aldol Cyclization of 1,2-Diketones in Carbohydrate Systems: Stereoselective Synthesis of Cyclopentitols†
Dimitri Álvarez-Dorta
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorElisa I. León Dr.
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorAlan R. Kennedy Dr.
WestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow G1 1XL, Scotland (UK)
Search for more papers by this authorConcepción Riesco-Fagundo Dr.
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorErnesto Suárez Prof. Dr.
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorDimitri Álvarez-Dorta
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorElisa I. León Dr.
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorAlan R. Kennedy Dr.
WestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow G1 1XL, Scotland (UK)
Search for more papers by this authorConcepción Riesco-Fagundo Dr.
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorErnesto Suárez Prof. Dr.
Instituto de Productos Naturales y Agrobiología del CSIC, Carretera de La Esperanza 3, 38206 La Laguna, Tenerife (Spain), Fax: (+34) 922-260-135
Search for more papers by this authorThis research was supported by the Investigation Program (nos. CTQ2004-06381/BQU, CTQ2004-02367/BQU, and CTQ2007-67492/BQU) of the Ministerio de Educación y Ciencia, Spain and cofinanced by the Fondo Europeo de Desarrollo Regional (FEDER). D.A.-D. and C.R.-F. thank the Ministerio de Ciencia e Innovación, Spain and the I3P-CSIC Program, respectively, for fellowships.
Graphical Abstract
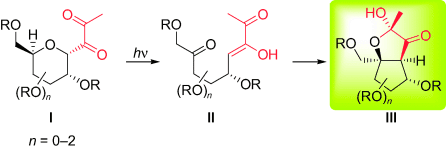
Opened and closed: Visible-light photostimulation of 2,3-diuloses I triggers an unprecedented sequential rearrangement: A Norrish type II photoelimination to give an isolable acyclic photoenol intermediate II is followed by an intramolecular enolexo aldolization. The contraction of the pyranose ring in this process leads to a new type of cyclopentitol derivative III. R=acyl, alkyl, silyl group.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200803696_sm_miscellaneous_information.pdf309.5 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on the Norrish–Yang reaction, see:
- 1a Synthetic Organic Photochemistry (Molecular and Supramolecular Photochemistry), Vol. 12 (Eds.: ), Marcel Dekker, New York, 2005, pp. 11–39 and 41–87;
- 1b Handbook of Organic Photochemistry and Photobiology, Vol. 1, 2nd ed. ), CRC, Boca Raton, 2003, chap. 52, 55, 57, and 58;
- 1cP. J. Wagner, B.-S. Park in Organic Photochemistry, Vol. 11 (Ed.: ), Marcel Dekker, New York, 1991, pp. 227–365;
- 1dP. J. Wagner, Acc. Chem. Res. 1989, 22, 83–91;
- 1eM. B. Rubin, Top. Curr. Chem. 1985, 129, 1–56.
- 2
- 2aN. C. Yang, D.-D. H. Yang, J. Am. Chem. Soc. 1958, 80, 2913–2914;
- 2bW. H. Urry, D. J. Trecker, J. Am. Chem. Soc. 1962, 84, 118–120.
- 3For the Norrish type II photoelimination of 1,2-diketones, see:
- 3aR. Bishop, J. Chem. Soc. Chem. Commun. 1972, 1288–1289;
- 3bS. Mohr, Tetrahedron Lett. 1980, 21, 593–594.
- 4
- 4aN. K. Hamer, Tetrahedron Lett. 1982, 23, 473–474;
- 4bN. K. Hamer, J. Chem. Soc. Perkin Trans. 1 1983, 61–64;
- 4cN. K. Hamer, Tetrahedron Lett. 1986, 27, 2167–2168;
- 4dM. Obayashi, E. Mizuta, S. Noguchi, Chem. Pharm. Bull. 1979, 27, 1679–1682.
- 5N. J. Turro, T.-J. Lee, J. Am. Chem. Soc. 1969, 91, 5651–5652.
- 6A. J. Herrera, M. Rondón, E. Suárez, J. Org. Chem. 2008, 73, 3384–3391.
- 7
- 7aA. Martín, I. Pérez-Martín, L. M. Quintanal, E. Suárez, Org. Lett. 2007, 9, 1785–1788;
- 7bC. G. Francisco, A. J. Herrera, A. R. Kennedy, D. Melián, E. Suárez, Angew. Chem. 2002, 114, 884–886;
10.1002/1521-3757(20020301)114:5<884::AID-ANGE884>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2002, 41, 856–858.10.1002/1521-3773(20020301)41:5<856::AID-ANIE856>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 8For a review on the photochemistry of carbohydrates, see: G. Descotes, Top. Curr. Chem. 1990, 154, 39–76.
- 9C. G. Francisco, A. J. Herrera, E. Suárez, J. Org. Chem. 2002, 67, 7439–7445.
- 10
- 10aC. G. Francisco, R. Freire, A. J. Herrera, I. Pérez-Martín, E. Suárez, Org. Lett. 2002, 4, 1959–1961;
- 10bC. G. Francisco, A. J. Herrera, E. Suárez, Tetrahedron Lett. 2000, 41, 7869–7873.
- 11
- 11aP. S. Bailey, Chem. Rev. 1958, 58, 925–1010;
- 11bT. F. Favino, G. Fronza, C. Fuganti, D. Fuganti, P. Graselli, A. Mele, J. Org. Chem. 1996, 61, 8975–8979;
- 11cL. Re, B. Maurer, G. Ohloff, Helv. Chim. Acta 1973, 56, 1882–1894.
- 12
- 12aM. Schröder, Chem. Rev. 1980, 80, 187–213;
- 12bR. Zibuck, D. Seebach, Helv. Chim. Acta 1988, 71, 237–240.
- 13Daylight lamp refers to the Philips master PL electronic lamp 23W/865. Irradiation outdoors with sunlight gave similar results.
- 14CCDC 695280 (7) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15
- 15a Modern Aldol Reactions, Vols. 1–2 (Ed.: ), Wiley-VCH, Weinheim, 2004; for reviews on the synthesis of cyclopentitols by aldolization in carbohydrate chemistry, see:
- 15bO. Arjona, A. M. Gómez, J. C. López, J. Plumet, Chem. Rev. 2007, 107, 1919–2036;
- 15cM. Sollogoub, P. Sinaÿ in The Organic Chemistry of Sugars (Eds.: ), CRC, Boca Raton, 2006, pp. 349–381;
- 15dP. I. Dalko, P. Sinaÿ, Angew. Chem. 1999, 111, 819–823;
10.1002/(SICI)1521-3757(19990315)111:6<819::AID-ANGE819>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 1999, 38, 773–777;10.1002/(SICI)1521-3773(19990315)38:6<773::AID-ANIE773>3.0.CO;2-N CAS Web of Science® Google Scholar
- 15eR. J. Ferrier, S. Middleton, Chem. Rev. 1993, 93, 2779–2831.
- 165-Hydroxy-2,3-pentanedione (laurencione), a naturally occurring 1,2-diketone isolated from the red alga Laurencia spectabilis, also exists preferentially in a hemiacetal form (2-hydroxy-2-methyldihydro-3(2H)-furanone):
- 16aM. W. Bernart, W. H. Gerwick, E. E. Corcoran, A. Y. Lee, J. Clardy, Phytochemistry 1992, 31, 1273–1276; for a synthesis, see:
- 16bN. De Kimpe, A. Georgieva, M. Boeykens, L. Lazar, J. Org. Chem. 1995, 60, 5262–5265.
- 17
- 17aP. J. Wagner, B.-S. Park, M. Sobczak, J. Frey, Z. Rappoport, J. Am. Chem. Soc. 1995, 117, 7619–7629;
- 17bP. J. Wagner, Y. Zhang, A. E. Puchalski, J. Phys. Chem. 1993, 97, 13368–13374;
- 17cA. Demeter, T. Bérces, J. Phys. Chem. 1991, 95, 1228–1232;
- 17dY. M. A. Naguib, C. Steel, S. G. Cohen, J. Phys. Chem. 1988, 92, 6574–6579.
- 18Similarly, the 1H NMR signal for the vinyl hydrogen atom of a photoenol obtained by irradiation of 4,4-dimethyl-1-phenyl-1,2-pentanedione was reported to appear at δ=5.35 ppm: P. J. Wagner, R. G. Zepp, K.-C. Liu, M. Thomas, T.-J. Lee, N. J. Turro, J. Am. Chem. Soc. 1976, 98, 8125–8134.
- 19For reviews on photoenolization processes, see:
- 19aP. G. Sammes, Tetrahedron 1976, 32, 405–422;
- 19bJ. L. Charlton, M. M. Alauddin, Tetrahedron 1987, 43, 2873–2889; for a related photoenolization/Diels–Alder sequence, see:
- 19cN. Yang, C. Rivas, J. Am. Chem. Soc. 1961, 83, 2213;
- 19dK. C. Nicolaou, D. L. F. Gray, J. Tae, J. Am. Chem. Soc. 2004, 126, 613–627.
- 20For diketones 1–4, a word of caution is required in regard to the observed syn stereoselectivity of the aldol cyclization. Minor stereoisomers with an unprotected 1,2-diketone moiety (anti aldols) could be destroyed by secondary photolysis.
- 21Compounds 15 and 16 resemble cyclopentitols obtained by SmI2-promoted pinacol-coupling cyclization of 1,5-diulose compounds derived from D-glucose:
- 21aI. Storch de Gracia, H. Dietrich, S. Bobo, J. L. Chiara, J. Org. Chem. 1998, 63, 5883–5889;
- 21bA. Boiron, P. Zillig, D. Faber, B. Giese, J. Org. Chem. 1998, 63, 5877–5882.
- 22
- 22aP. de Armas, C. G. Francisco, E. Suárez, Angew. Chem. 1992, 104, 746–748; Angew. Chem. Int. Ed. Engl. 1992, 31, 772–774;
- 22bC. C. González, A. R. Kennedy, E. I. León, C. Riesco-Fagundo, E. Suárez, Angew. Chem. 2001, 113, 2388–2390;
Angew. Chem. Int. Ed. 2001, 40, 2326–2328.
10.1002/1521-3773(20010618)40:12<2326::AID-ANIE2326>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar