[(NHC)2Cu]X Complexes as Efficient Catalysts for Azide–Alkyne Click Chemistry at Low Catalyst Loadings†
Silvia Díez-González Dr.
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-244 http://www.iciq.es/english/research/grups_eng/nolan/entrada.htm
Search for more papers by this authorSteven P. Nolan Prof. Dr.
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-244 http://www.iciq.es/english/research/grups_eng/nolan/entrada.htm
Search for more papers by this authorSilvia Díez-González Dr.
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-244 http://www.iciq.es/english/research/grups_eng/nolan/entrada.htm
Search for more papers by this authorSteven P. Nolan Prof. Dr.
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-244 http://www.iciq.es/english/research/grups_eng/nolan/entrada.htm
Search for more papers by this authorThe ICIQ Foundation and the Spanish Ministerio de Educación y Ciencia are gratefully acknowledged for financial support. S.D.G. thanks the MEC for financial support through the Torres Quevedo program. S.P.N. is an ICREA Research Professor. We thank Dr. Nicolas “La Click, c'est chic” Marion for insightful reading of this manuscript. NHC=N-heterocyclic carbene.
Graphical Abstract
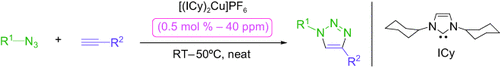
La click, c'est chic! A catalytic system based on an [(NHC)2Cu]X complex (NHC=N-heterocyclic carbene) was developed for the [3+2] cycloaddition of azides with alkynes under click conditions (see scheme). This system is broad in scope and highly efficient (turnover frequencies up to 5000 h−1) even at very low catalyst loadings (down to 40 ppm). Preliminary mechanistic studies suggest a specific precatalyst-activation pathway.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200803289_sm_miscellaneous_information.pdf1.3 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. 2001, 113, 2056–2075;
Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 2
- 2aC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057–3064;
- 2bV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708–2711;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596–2599.10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 3
- 3aR. Huisgen, Pure Appl. Chem. 1989, 61, 613–628;
- 3bA. Padwa in Comprehensive Organic Synthesis, Vol. 4 (Ed.: ), Pergamon, Oxford, 1991, pp. 1069–1109.
10.1016/B978-0-08-052349-1.00116-5 Google Scholar
- 4For reviews, see:
- 4aJ. E. Moses, A. D. Moorhouse, Chem. Soc. Rev. 2007, 36, 526–539;
- 4bA. Dondoni, Chem. Asian J. 2007, 2, 700–708;
- 4cJ.-F. Lutz, Angew. Chem. 2007, 119, 1036–1043;
10.1002/ange.200604050 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1018–1025;
- 4dSpecial issue: Click Chemistry, QSAR Comb. Sci. 2007, 26, 1110–1323.
- 5
- 5aT. R. Chan, R. Hilgraf, K. B. Sharpless, V. V. Fokin, Org. Lett. 2004, 6, 2853–2855;
- 5bV. O. Rodionov, S. I. Presolsky, S. Gardinier, Y.-H. Lim, M. G. Finn, J. Am. Chem. Soc. 2007, 129, 12696–12704;
- 5cS. S. Gupta, J. Kuzelka, P. Singh, W. G. Lewis, M. Manchester, M. G. Finn, Bioconjugate Chem. 2005, 16, 1572–1579.
- 6F. Pérez-Balderas, M. Ortega-Muñoz, J. Morales-Sanfrutos, F. Hernández-Mateo, F. G. Calvo-Flores, J. A. Calvo-Asín, J. Isac-García, F. Santoyo-González, Org. Lett. 2003, 5, 1951–1954.
- 7B. Gerard, J. Ryan, A. B. Beeler, J. A. Porco, Jr., Tetrahedron 2006, 62, 6405–6411.
- 8N. Candelon, D. Lastécouères, A. K. Diallo, J. Ruiz Aranzaes, D. Astruc, J.-M. Vincent, Chem. Commun. 2008, 741–743.
- 9S. Díez-González, A. Correa, L. Cavallo, S. P. Nolan, Chem. Eur. J. 2006, 12, 7558–7564.
- 10J. Broggi, S. Díez-González, J. L. Petersen, S. Berteina-Raboin, S. P. Nolan, L. A. Agrofoglio, Synthesis 2008, 141–148.
- 11M. Séverac, L. Le Pleux, A. Scarpaci, E. Blart, F. Odobel, Tetrahedron Lett. 2007, 48, 6518–6522.
- 12A. Maisonial, P. Serafin, M. Traïkia, E. Debiton, V. Théry, D. J. Aitken, P. Lemoine, B. Voissat, A. Gautier, Eur. J. Inorg. Chem. 2008, 298–305.
- 13
- 13aS. Díez-González, E. D. Stevens, S. P. Nolan, Chem. Commun. 2008, 4747–4749.
- 14
- 14aS. Díez-González, N. M. Scott, S. P. Nolan, Organometallics 2006, 25, 2355–2358;
- 14bS. Díez-González, E. D. Stevens, N. M. Scott, J. L. Petersen, S. P. Nolan, Chem. Eur. J. 2008, 14, 158–168.
- 15
- 15aH. Kaur, F. K. Zinn, E. D. Stevens, S. P. Nolan, Organometallics 2004, 23, 1157–1160;
- 15bS. Díez-González, H. Kaur, F. K. Zinn, E. D. Stevens, S. P. Nolan, J. Org. Chem. 2005, 70, 4784–4796.
- 16For discussion on the stereoelectronic parameters of NHC ligands, see:
- 16aT. Strassner, Top. Organomet. Chem. 2004, 13, 1–20;
- 16bL. Cavallo, A. Correa, C. Costabile, H. Jacobsen, J. Organomet. Chem. 2005, 690, 5407–5413;
- 16cS. Díez-González, S. P. Nolan, Coord. Chem. Rev. 2007, 251, 874–883.
- 17During the optimization studies, complete conversion was observed in dimethyl sulfoxide (2 h), water (1.5 h), N,N-dimethylformamide (1.5 h), THF (1 h), acetone (0.5 h), and MeCN (0.2 h) in the presence of 5 a (2 mol %). Sluggish reactions were observed when alcohols were used as solvents.
- 18For reviews, see:
- 18aS. Díez-González, S. P. Nolan, Synlett 2007, 2158–2167;
- 18bS. Díez-González, S. P. Nolan, Aldrichimica Acta 2008, 41, 43–51.
- 19For example, triazole 8 g was produced quantitatively at 40 °C in 3 h instead of 9 h. For triazoles 8 n and 8 o, a reaction time of only 1.5 h was required at 40 °C.
- 20For further details, see the Supporting Information.
- 21Vincent and co-workers recently reported a copper(I)–tren catalyst (tren=tris(2-aminoethylamine)) that promoted the Huisgen cycloaddition with high turnover numbers (TONs). TON values of up to 54 000 were observed for the preparation of 8 a at 60 °C in 24 h in the absence of a solvent.[8] However, during our studies, even for the formation of the same triazole, we observed the non-negligible formation of the 1,5-regioisomer at 50 °C after only 5 h.
- 22F. Liu, W. Chen, X. You, J. Chem. Crystallogr. 2002, 32, 27–31.
- 23L. A. Goj, E. D. Blue, C. Munro-Leighton, T. B. Gunnoe, J. L. Petersen, Inorg. Chem. 2005, 44, 8647–8649.
- 24F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless, V. V. Fokin, J. Am. Chem. Soc. 2004, 127, 210–216.
- 25For a review, see: V. D. Bock, H. Hiemstra, J. H. van Maarseveen, Eur. J. Org. Chem. 2006, 51–68; see also:
- 25aB. F. Straub, Chem. Commun. 2007, 3868–3870;
- 25bM. Ahlquist, V. V. Fokin, Organometallics 2007, 26, 4389–4391.
- 26C. Nolte, P. Mayer, B. F. Straub, Angew. Chem. 2007, 119, 2147–2149;
10.1002/ange.200604444 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2101–2103; for a stable gold–triazolide complex, see: D. V. Partyka, J. B. Updegraff III, M. Zeller, A. D. Hunter, T. G. Gray, Organometallics 2007, 26, 183–186.