Stable Deacon Process for HCl Oxidation over RuO2†
H.O. acknowledges the DFG for financial support (Ov21/7) and the Leibniz-Rechenzentrum in Munich for providing us with parallel computing time. E.L. and J.N.A. thank for financial support by the Swedish Research Council. C.J.W. thanks for financial support from the Netherlands Organisation for Scientific research through the Rubicon program.
Graphical Abstract
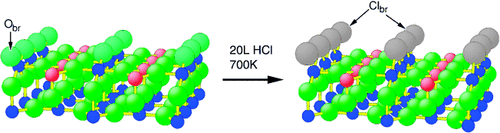
Selective substitution: In the oxidation of HCl with oxygen to give Cl2 and water, RuO2(110) serves as a stable, active model catalyst for the Sumitomo process (see picture; Ru in red and blue). The stability of the catalyst is related to the selective replacement of undercoordinated bridging surface O atoms (Obr) by Cl atoms (Clbr). The chlorination of RuO2(110) is self-limiting, in that chlorine incorporation terminates when all bridging O atoms are replaced.