A Self-Assembled Spin Cage
Koji Nakabayashi
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorYusuke Ozaki
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorMasaki Kawano Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorMakoto Fujita Prof. Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorKoji Nakabayashi
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorYusuke Ozaki
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorMasaki Kawano Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorMakoto Fujita Prof. Dr.
Department of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan, Fax: (+81) 3-5841-7257
Search for more papers by this authorGraphical Abstract
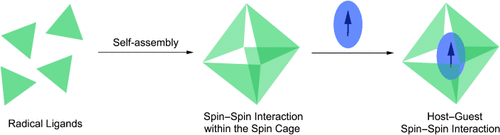
Going for a spin: The quantitative self-assembly of a verdazyl radical-cored ligand with [Pd(bpy)(NO3)2] (bpy=2,2′-bipyridyl) generates a large M6L4 spin cage in which multiple spin centers effectively surround the cavity. The inclusion of a radical guest within the cavity induces spin–spin interactions between the host and the guest.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z704924_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Self-assembled ionic hosts:
- 1aM. Fujita, D. Oguro, M. Miyazawa, H. Oka, K. Yamaguchi, K. Ogura, Nature 1995, 378, 469–472;
- 1bD. L. Caulder, K. N. Raymond, Acc. Chem. Res. 1999, 32, 975–982;
- 1cS. Leininger, B. Olenyuk, P. J. Stang, Chem. Rev. 2000, 100, 853–908;
- 1dM. Fujita, K. Umemoto, M. Yoshizawa, N. Fujita, T. Kusukawa, K. Biradha, Chem. Commun. 2001, 509–518;
- 1eF. A. Cotton, C. Lin, C. A. Murillo, Acc. Chem. Res. 2001, 34, 759–771;
- 1fF. Hof, S. L. Craig, C. Nuckolls, J. Rebek, Jr., Angew. Chem. 2002, 114, 1556–1578;
10.1002/1521-3757(20020503)114:9<1556::AID-ANGE1556>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1488–1508;10.1002/1521-3773(20020503)41:9<1488::AID-ANIE1488>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 1gM. Fujita, M. Tominaga, A. Hori, B. Therrien, Acc. Chem. Res. 2005, 38, 369–378;
- 1hM. D. Pluth, R. G. Bergman, K. N. Raymond, J. Am. Chem. Soc. 2007, 129, 11459–11467.
- 2Cagelike compounds with spins at metal centers: Reviews:
- 2aE. J. L. McInnes, S. Piligkos, G. A. Timco, R. E. P. Winpenny, Coord. Chem. Rev. 2005, 249, 2577–2590;
- 2bG. Christou, Polyhedron 2005, 24, 2065–2075; radical cages:
- 2cT. Okubo, S. Kitagawa, M. Kondo, H. Matsuzaka, T. Ishii, Angew. Chem. 1999, 111, 980–983;
Angew. Chem. Int. Ed. 1999, 38, 931–933;
10.1002/(SICI)1521-3773(19990401)38:7<931::AID-ANIE931>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 2dL. Kröck, A. Shivanyuk, D. B. Goodin, J. Rebek, Jr., Chem. Commun. 2004, 272–273;
- 2eA. Rajca, S. Mukherjee, M. Pink, S. Rajca, J. Am. Chem. Soc. 2006, 128, 13497–13507;
- 2fK. Igarashi, T. Nogamia, T. Ishida, Chem. Commun. 2007, 501–503.
- 3
- 3aT. Kusukawa, M. Fujita, J. Am. Chem. Soc. 2002, 124, 13576–13582;
- 3bM. Yoshizawa, M. Tamura, M. Fujita, J. Am. Chem. Soc. 2004, 126, 6846–6847.
- 4
- 4aR. Kuhn, F. A. Neugebauer, H. Trischmann, Monatsh. Chem. 1966, 97, 525–553;
- 4bY. A. Ibrahim, A. H. M. Elwahy, A. A. Abbas, Tetrahedron 1994, 50, 11489–11498; detailed conditions are described in the Supporting Information.
- 5The CSI-MS spectrum of 2 (PF6 salt) and the ESR spectra of 2 recorded at several microwave powers are shown in Figures S1 and S2, respectively, of the Supporting Information.
- 6The titration experiment of radical ligand 1 into [Pd(bpy)(NO3)2] is shown in Figure S3 of the Supporting Information.
- 7The quality of the crystals was improved by adding 1-adamantanol as a template.
- 8The ESR spectra of 2 recorded at different concentrations and temperatures are shown in Figures S4 and S5 of the Supporting Information.
- 9J. A. Weil, J. R. Bolton, Electron Paramagnetic Resonance. Elementary Theory and Practical Applications, 2nd ed., Wiley-Intersciene, 2007, pp. 322–324.
- 10The nitrosyl radicals 3 and 4 that were used as guests were prepared by the improved Ullman's procedure described in the following reference: C. Hirel, K. E. Vostrikova, J. Pecaut, V. I. Ovcharenko, P. Rey, Chem. Eur. J. 2001, 7, 2007–2014.
10.1002/1521-3765(20010504)7:9<2007::AID-CHEM2007>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 112⋅3: Elemental analysis calcd for C128H104N52O36Pd6⋅C21H21N2O2⋅21 H2O: C 41.65, H 3.92, N 17.60; found: C 41.75, H 4.04, N 17.38. 2⋅(4)2: Elemental analysis calcd for C128H104N52O36Pd6⋅2 C17H19N2O2⋅22 H2O: C 42.78, H 4.12, N 17.25; found: C 42.82, H 4.10, N 17.11. The extraction experiments are described in Section 7 of the Supporting Information.
- 12The χT versus T plots of 2 and 2⋅3 are shown in Figure S6 of the Supporting Information.
- 13
- 13aK. Nakabayashi, M. Kawano, M. Yoshizawa, S. Ohkoshi, M. Fujita, J. Am. Chem. Soc. 2004, 126, 16694–16695;
- 13bK. Nakabayashi, M. Kawano, T. Kato, K. Furukawa, S. Ohkoshi, T. Hozumi, M. Fujita, Chem. Asian J. 2007, 2, 164–170.
- 14The enclathration of neutral molecules is described in the Supporting Information.