(N-Heterocyclic Carbene)Gold(I)-Catalyzed Cycloisomerization of Cyclohexadienyl Alkynes to Tetracyclo[3.3.0.02,8.04,6]octanes†
Soo Min Kim
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorJi Hoon Park
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorSoo Young Choi
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorYoung Keun Chung Prof.
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorSoo Min Kim
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorJi Hoon Park
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorSoo Young Choi
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorYoung Keun Chung Prof.
Intelligent Textile System Research Center, Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 151-747, Korea, Fax: (+82) 2-889-0310
Search for more papers by this authorThis work was supported by the Korea Research Foundation grant funded by the Korean Government (MOEHRD) (R02-2004-000-10005–0 and KRF-2005-070-C00072) and the SRC/ERC program of MOST/KOSEF (R11-2005-065). S.M.K., J.H.P., and S.Y.C. were supported by Brain Korea 21 Fellowships and Seoul Science Fellowships.
Graphical Abstract
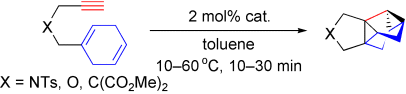
Biscyclopropanation: Dienynes containing a cyclohexadienyl unit can be converted into tetracyclo[3.3.0.02,8.04,6]octanes by treatment with an (N-heterocyclic carbene)gold catalyst. When the dienynes bear an open-chain diene instead of cyclohexadiene, open-cage compounds, with two sides missing from the cage, are obtained.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z702028_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. C. Lloyd-Jones, Org. Biomol. Chem. 2003, 1, 215;
- 1bM. Méndez, V. Mamane, A. Fürstner, Chemtracts 2003, 16, 397;
- 1cC. Aubert, O. Buisine, M. Malacria, Chem. Rev. 2002, 102, 813.
- 2For reviews, see:
- 2aL. M. Zhang, J. W. Sun, S. A. Kozmin, Adv. Synth. Catal. 2006, 348, 2271;
- 2bC. Nieto-Oberhuber, S. López, E. Jiménez-Núñez, A. M. Echavarren, Chem. Eur. J. 2006, 12, 5916;
- 2cC. Bruneau, Angew. Chem. 2005, 117, 2380;
10.1002/ange.200462568 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2328;
- 2dI. J. S. Fairlamb, Angew. Chem. 2004, 116, 1066;
10.1002/ange.200301699 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1048.
- 3For recent gold-catalyzed reactions, see:
- 3aA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. 2006, 118, 8064;
10.1002/ange.200602454 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7896;
- 3bG. Lemière, V. Gahdon, N. Agenet, J. P. Goddard, A. de Kozak, C. Aubert, L. Fensterbank, M. Malacria, Angew. Chem. 2006, 118, 6878;
10.1002/ange.200602189 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7596;
- 3cS. Wang, L. Zhang, J. Am. Chem. Soc. 2006, 128, 14274;
- 3dS. Couty, C. Meyer, J. Cossy, Angew. Chem. 2006, 118, 6878;
10.1002/ange.200602270 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6726.
- 4For recent papers, see:
- 4aJ. Marco-Contelles, N. Arroyo, S. Anjum, E. Mainetti, N. Marion, K. Cariou, G. Lemière, V. Mouriès, L. Fensterbank, M. Malacria, Eur. J. Org. Chem. 2006, 4618;
- 4bS. I. Lee, S. M. Kim, S. Y. Kim, Y. K. Chung, Synlett 2006, 2256;
- 4cY. Miyanohana, N. Chatani, Org. Lett. 2006, 8, 2155.
- 5For Pt-catalyzed reactions, see:
- 5aA. Fürstner, F. Stelzer, H. Szillat, J. Am. Chem. Soc. 2001, 123, 11863. For Fe0-catalyzed reactions, see:
- 5bA. Fürstner, R. Martin, K. Majima, J. Am. Chem. Soc. 2005, 127, 12236. For Rh-catalyzed reactions, see:
- 5cH. Kim, C. Lee, J. Am. Chem. Soc. 2005, 127, 10180.
- 6
- 6aS. M. Kim, S. I. Lee, Y. K. Chung, Org. Lett. 2006, 8, 5425;
- 6bS. I. Lee, S. M. Kim, M. R. Choi, S. Y. Kim, Y. K. Chung, J. Org. Chem. 2006, 71, 9366.
- 7For recent reviews, see:
- 7aP. L. Arnold, S. T. Liddle, Chem. Commun. 2006, 3959;
- 7bR. H. Crabtree, J. Organomet. Chem. 2005, 690, 5451;
- 7cV. César, S. Bellemin-Laponnaz, L. H. Gade, Chem. Soc. Rev. 2004, 33, 619;
- 7dC. M. Crudden, D. P. Allen, Coord. Chem. Rev. 2004, 248, 2247;
- 7eE. Peris, R. H. Crabtree, Coord. Chem. Rev. 2004, 248, 2239.
- 8
- 8aL. Ray, V. Katiyar, M. J. Raihan, H. Nanavati, M. M. Shaikh, P. Ghosh, Eur. J. Inorg. Chem. 2006, 3724;
- 8bN. Marion, P. de Frémont, G. Lemière, E. D. Stevens, L. Fensterbank, M. Malacria, S. P. Nolan, Chem. Commun. 2006, 2048;
- 8cM. R. Fructos, T. R. Belderrain, P. de Fremont, N. M. Scott, S. P. Nolan, M. M. Diaz-Requejo, P. J. Perez, Angew. Chem. 2005, 117, 5418;
10.1002/ange.200501056 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5284.
- 9
- 9aN. A. LeBel, R. Liesemer, J. Am. Chem. Soc. 1965, 87, 4301;
- 9bI. Erden, Chem. Lett. 1981, 263;
- 9cP. K. Freeman, K. E. Swenson, J. Org. Chem. 1982, 47, 2033. For a catalytic reaction, see:
- 9dH. C. Volger, H. Hogeveen, M. M. P. Gaasbeek, J. Am. Chem. Soc. 1969, 91, 2137.
- 10T. A. Zeidan, S. V. Kovalenko, M. Manoharan, R. J. Clark, I. Ghiviriga, I. V. Alabugin, J. Am. Chem. Soc. 2005, 127, 4270.
- 11The synthetic procedures and spectroscopic data of the new compounds are summarized in the Supporting Information. CCDC- 645350 (1 b) and CCDC-645351 (7 d) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12T. Shibata, Y.-K. Tahara, J. Am. Chem. Soc. 2006, 128, 11766.
- 13For pioneering work on 1,6-enyne cycloisomerization involving cyclopropyl gold carbenoids, see: C. Nieto-Oberhuber, M. P. Muñoz, E. Buñuel, C. Nevado, D. J. Cárdenas, A. M. Echavarren, Angew. Chem. 2004, 116, 2456;
10.1002/ange.200353207 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2402. The authors appreciate one of referees' suggestion (i.e., the involvement of intermediate II in Scheme 1) to explain the formation of b and d.