Dynamic Kinetic Asymmetric Allylic Amination and Acyl Migration of Vinyl Aziridines with Imido Carboxylates†
We thank the National Science Foundation and the National Institutes of Health, General Medical Sciences Institute (GM-13598), for their generous support of our programs. Mass spectra were provided by the Mass Spectrometry Facility of the University of California-San Francisco, supported by NIH Division of Research Resources.
Graphical Abstract
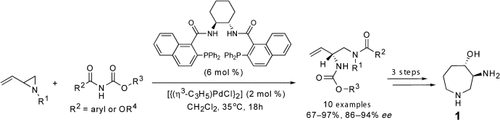
An atom-economical method has been developed for the preparation of chiral vicinal diamines through a dynamic kinetic asymmetric allylic amination and acyl-group migration of vinyl aziridines with imido carboxylates. Application of the asymmetric transformation enabled the concise synthesis of the azepane core 1 of (+)-balanol and its syn analogue.