Azoporphyrin: The Porphyrin Analogue of Azobenzene†
Louisa J. Esdaile
School of Physical and Chemical Sciences, Queensland University of Technology, G.P.O. Box 2434, Brisbane 4001, Australia, Fax: (+61) 7-3138-1804
Search for more papers by this authorPaul Jensen Dr.
Crystal Structure Analysis Facility, School of Chemistry, University of Sydney, N.S.W. 2006, Australia
Search for more papers by this authorJohn C. McMurtrie Dr.
School of Physical and Chemical Sciences, Queensland University of Technology, G.P.O. Box 2434, Brisbane 4001, Australia, Fax: (+61) 7-3138-1804
Search for more papers by this authorDennis P. Arnold Dr.
School of Physical and Chemical Sciences, Queensland University of Technology, G.P.O. Box 2434, Brisbane 4001, Australia, Fax: (+61) 7-3138-1804
Search for more papers by this authorLouisa J. Esdaile
School of Physical and Chemical Sciences, Queensland University of Technology, G.P.O. Box 2434, Brisbane 4001, Australia, Fax: (+61) 7-3138-1804
Search for more papers by this authorPaul Jensen Dr.
Crystal Structure Analysis Facility, School of Chemistry, University of Sydney, N.S.W. 2006, Australia
Search for more papers by this authorJohn C. McMurtrie Dr.
School of Physical and Chemical Sciences, Queensland University of Technology, G.P.O. Box 2434, Brisbane 4001, Australia, Fax: (+61) 7-3138-1804
Search for more papers by this authorDennis P. Arnold Dr.
School of Physical and Chemical Sciences, Queensland University of Technology, G.P.O. Box 2434, Brisbane 4001, Australia, Fax: (+61) 7-3138-1804
Search for more papers by this authorWe thank the Australian Research Council for Discovery Grant DP0663774, and the Faculty of Science, Queensland University of Technology for a Postgraduate Scholarship for L.J.E.
Graphical Abstract
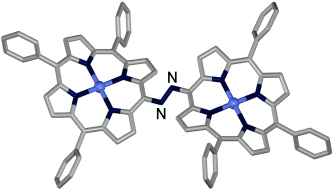
Excellent conjugation: The novel azoporphyrins (1,2-bis(porphyrinyl)diazenes) have been prepared by using copper-catalyzed coupling of primary amines. The structure of the azo(triphenylporphyrin) was determined by X-ray crystallography (see picture). The azo linker provides an excellent conjugating pathway for expansion of porphyrin π conjugation.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z604658_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For some recent applications of azobenzenes, see:
- 1aS. Loudwig, H. Bayley, J. Am. Chem. Soc. 2006, 128, 12404;
- 1bM. L. Bossi, D. H. Murgida, P. F. Aramendia, J. Phys. Chem. B 2006, 110, 13804;
- 1cS. Yagai, T. Iwashima, K. Kishikawa, S. Nakahara, T. Karatsu, A. Kitamura, Chem. Eur. J. 2006, 12, 3984;
- 1dT. Yamamato, Y. Umemura, O. Sato, Y. Einaga, Sci. Technol. Adv. Mater. 2006, 7, 134;
- 1eD. Takamatsu, Y. Yamakoshi, K.-i. Fukui, J. Phys. Chem. B 2006, 110, 1968; E. J. Harbron, D. A. Vicente, D. H. Hadley, M. R. Imm, J. Phys. Chem. A 2005, 109, 10846.
- 2
- 2aJ.-M. Lehn, Chem. Eur. J. 2006, 12, 5910;
- 2bG. S. Kottas, L. I. Clarke, D. Horinek, J. Michl, Chem. Rev. 2005, 105, 1281;
- 2cK. Kinbara, T. Aida, Chem. Rev. 2005, 105, 1377.
- 3
- 3aT. Muraoka, K. Kinbara, T. Aida, Nature 2006, 440, 512;
- 3bD. Gust, T. A. Moore, A. L. Moore, Chem. Commun. 2006, 1169;
- 3cJ. L. Rodríguez-Redondo, A. Sastre-Santos, F. Fernández-Lázaro, D. Soares, G. C. Azzellini, B. Elliott, L. Echegoyen, Chem. Commun. 2006, 1265.
- 4
- 4aT. Ikeda, J. M. Lintuluoto, N. Aratani, Z. S. Yoon, D. Kim, A. Osuka, Eur. J. Org. Chem. 2006, 3193;
- 4bH. S. Cho, D. H. Jeong, S. Cho, D. Kim, Y. Matsuzaki, K. Tanaka, A. Tsuda, A. Osuka, J. Am. Chem. Soc. 2002, 124, 14642.
- 5
- 5aM. Chachisvilis, V. S. Chirvony, A. M. Shulga, B. Källebring, S. Larsson, V. Sundström, J. Phys. Chem. 1996, 100, 13857;
- 5bE. Blart, F. Suzunet, J.-P. Quintard, F. Odobel, J. Porphyrins Phthalocyanines 2003, 7, 207;
- 5cM. J. Frampton, H. Akdas, A. R. Cowley, J. E. Rogers, J. E. Slagle, P. A. Fleitz, M. Drobizhev, A. Rebane, H. L. Anderson, Org. Lett. 2005, 7, 5365;
- 5dO. B. Locos, D. P. Arnold, Org. Biomol. Chem. 2006, 4, 902.
- 6For some examples, see:
- 6aK. Susumu, P. R. Frail, P. J. Angiolillo, M. J. Therien, J. Am. Chem. Soc. 2006, 128, 8380;
- 6bK. Ogawa, H. Hasegawa, Y. Inaba, Y. Kobuke, H. Inouye, Y. Kanematsu, E. Kohno, T. Hirano, S. Ogura, I. Okura, J. Med. Chem. 2006, 49, 2276;
- 6cT.-H. Huang, Y.-J. Chen, S.-S. Lo, W.-N. Yen, C.-L. Mai, M.-C. Kuo, C.-Y. Yeh, Dalton Trans. 2006, 2207;
- 6dM. Drobizhev, Y. Stepanenko, Y. Dzenis, A. Karotki, A. Rebane, P. N. Taylor, H. L. Anderson, J. Am. Chem. Soc. 2004, 126, 15352;
- 6eD. P. Arnold, G. A. Heath, D. A. James, J. Porphyrins Phthalocyanines 1999, 3, 5;
- 6fG. J. Wilson, D. P. Arnold, J. Phys. Chem. A 2005, 109, 6104.
- 7T. E. O. Screen, I. M. Blake, L. H. Rees, W. Clegg, S. J. Borwick, H. L. Anderson, J. Chem. Soc. Perkin Trans. 1 2002, 320.
- 8For the combination of porphyrins or phthalocyanines with azoarenes, see:
- 8aM. V. Peters, R. Goddard, S. Hecht, J. Org. Chem. 2006, 71, 7846;
- 8bT. Yamamura, A. Momotake, T. Arai, Tetrahedron Lett. 2004, 45, 9219;
- 8cS. Tsuchiya, J. Am. Chem. Soc. 1999, 121, 48;
- 8dC. A. Hunter, L. D. Sarson, Tetrahedron Lett. 1996, 37, 699;
- 8eH. K. Hombrecher, K. Lüdtke, Tetrahedron 1993, 49, 9489.
- 9J. March, Advanced Organic Chemistry, 4th ed., Wiley, New York, 1992, p. 1280.
- 10K.-Y. Kim, J.-T. Shin, K.-S. Lee, C.-G. Cho, Tetrahedron Lett. 2004, 45, 117.
- 11L. J. Esdaile, J. C. McMurtrie, P. Turner, D. P. Arnold, Tetrahedron Lett. 2005, 46, 6931.
- 12L. J. Esdaile, M. O. Senge, D. P. Arnold, Chem. Commun. 2006, 4192.
- 13
- 13aK. Kinoshita, Bull. Chem. Soc. Jpn. 1959, 32, 780;
- 13bG. Engelsma, E. Havinga, Tetrahedron 1958, 2, 289;
- 13cE. G. Derouane, J. N. Braham, R. Hubin, J. Catal. 1974, 35, 196.
- 14Crystal data for Ni2-1 a⋅Py: C81H51N11Ni2, Mr=1295.75, monoclinic, P2/c, a=13.0693(3), b=9.7304(2), c=24.1344(5) Å, β=104.2410(10)°, V=2974.45(11) Å3, ρcalcd=1.447 g cm−3, Z=2, crystal size 0.20×0.13×0.09 mm, blue prism, T 150(2) K, λ(MoKα)=0.71073, μ(MoKα) 0.0.694 mm−1, Tmin/Tmax=0.885/0.944 (Gaussian absorption correction), 2θmax=59.96, hkl range −18 to 18, −13 to 13, −33 to 33, N=44 909, Nind=8570 (Rmerge=0.0266), Nobs=6976 (I>2σ(I)), Nvar=424, residuals R1(F, 2σ)=0.0423, wR2(F2, all)=0.0.0958, GoF(all)=1.023, Δρmin/Δρmax=−0.434/0.450 e Å−3. CCDC-627200 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15M. O. Senge, Chem. Commun. 2006, 243.
- 16These data are not directly comparable as the ethynediyl complex contains 5,15-bis(3,5-di-tert-butylphenyl)porphyrin (in benzene/pyridine 99:1), whereas the data for the ethenediyl and azo complexes pertain to 5,10,15-triphenylporphyrin structures (in CH2Cl2/pyridine 99:1).
- 17These data were measured by cyclic voltammetry in CH2Cl2/0.1 M Bu4NPF6, Pt working and counter electrodes, Ag/AgCl solid reference electrode, ferrocene at +0.55 V;
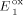 =+0.63 V,
=+0.63 V, 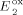 =+0.77 V,
=+0.77 V, 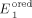 =−0.71 V,
=−0.71 V, 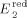 =−0.81 V.
=−0.81 V.
- 18Compound Ni2-5 was isolated in 26 % yield from reaction of Ni-3 b with 5,15-bis(3,5-di-tert-butylphenyl)porphyrinatonickel(II) and tert-butyl nitrite/BF3⋅Et2O in THF at RT; see: M. J. Smith, DPhil thesis, University of Oxford (UK), 2004 and H. L. Anderson, private communication.