Structure and Total Synthesis of Lysobactin (Katanosin B)†
Franz von Nussbaum Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorSonja Anlauf
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorJordi Benet-Buchholz Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
J.B.-B.: X-ray crystallography. L.M.: NMR solution structure.
Search for more papers by this authorDieter Häbich Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorJohannes Köbberling Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorLászló Musza Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
J.B.-B.: X-ray crystallography. L.M.: NMR solution structure.
Search for more papers by this authorJoachim Telser Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorHelga Rübsamen-Waigmann Prof. Dr.
AiCuris GmbH & Co. KG, Bayer Pharma- und Chemiepark, Friedrich-Ebert Strasse 475, 42117 Wuppertal, Germany
Search for more papers by this authorNina A. Brunner Dr.
AiCuris GmbH & Co. KG, Bayer Pharma- und Chemiepark, Friedrich-Ebert Strasse 475, 42117 Wuppertal, Germany
Search for more papers by this authorFranz von Nussbaum Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorSonja Anlauf
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorJordi Benet-Buchholz Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
J.B.-B.: X-ray crystallography. L.M.: NMR solution structure.
Search for more papers by this authorDieter Häbich Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorJohannes Köbberling Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorLászló Musza Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
J.B.-B.: X-ray crystallography. L.M.: NMR solution structure.
Search for more papers by this authorJoachim Telser Dr.
Bayer HealthCare AG, Medicinal Chemistry Europe, 42096 Wuppertal, Germany, Fax: (+49) 202-36-9694797
Search for more papers by this authorHelga Rübsamen-Waigmann Prof. Dr.
AiCuris GmbH & Co. KG, Bayer Pharma- und Chemiepark, Friedrich-Ebert Strasse 475, 42117 Wuppertal, Germany
Search for more papers by this authorNina A. Brunner Dr.
AiCuris GmbH & Co. KG, Bayer Pharma- und Chemiepark, Friedrich-Ebert Strasse 475, 42117 Wuppertal, Germany
Search for more papers by this authorWe are grateful to our colleagues C. Fehér, S. Heald, P. Schmitt, and S. Seip for NMR analyses. G. Schiffer determined the minimal inhibitory concentrations. A. Mayer-Bartschmidt and M.-A. Brüning carried out the fermentations. W. Schröder helped us with small-scale peptide analyses. J. Ragot resynthesized compound 2. We thank A. Göller, J. Schamberger, and M. Lobell for fruitful discussions about the 3D structure. We are indebted to H. Wild and H. Haning for their continuous support of this project and to H. Schirok and N. Griebenow for a critical revision of the manuscript.
Graphical Abstract
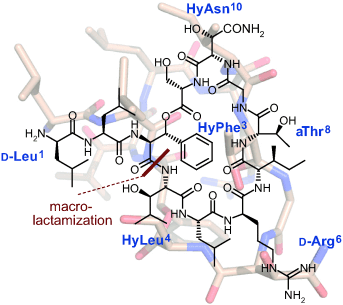
Tackle-resistant bacteria: Determination of the 3D structure of the antibiotic lysobactin has led to its total synthesis and resulted in a high-yielding macrolactamization step. The minimal use of protecting groups allowed preorganization of the side chains to steer the cyclization. Thus, a new chemical route has been developed in the search for innovative antibiotic lead structures.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z604232_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aInfectious Diseases Society of America (IDSA): http://www.idsociety.org;
- 1b46th Interscience Conference on Antimicrobial Agents and Chemotherapy 2006, San Francisco, USA: http://www.icaac.org.
- 2For a recent example (platensimycin), see:
- 2aJ. Wang et al., Nature 2006, 441, 358–361;
- 2bD. Häbich, F. von Nussbaum, ChemMedChem 2006, 1, 951–954;
- 2cK. C. Nicolaou, A. Li, D. J. Edmonds, Angew. Chem. 2006, 118, 7244–7248;
10.1002/ange.200603892 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7086–7090.
- 3L. Miesel, J. Greene, T. A. Black, Nat. Rev. Genet. 2003, 4, 442–456.
- 4F. von Nussbaum, M. Brands, B. Hinzen, S. Weigand, D. Häbich, Angew. Chem. 2006, 118, 5194–5254;
10.1002/ange.200600350 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5072–5129.
- 5F. Sarabia, S. Chammaa, A. S. Ruiz, L. M. Ortiz, F. J. López Herrera, Curr. Med. Chem. 2004, 11, 1309–1332.
- 6
- 6aD. P. Bonner, J. O'Sullivan, S. K. Tanaka, J. M. Clark, R. R. Whitney, J. Antibiot. 1988, 41, 1745–1751;
- 6bJ. O'Sullivan, J. E. McCullough, A. A. Tymiak, D. R. Kirsch, W. H. Trejo, P. A. Prinicipe, J. Antibiot. 1988, 41, 1740–1744.
- 7J. I. Shoji, H. Hinoo, K. Matsumoto, T. Hattori, T. Yoshida, S. Matsuura, E. Kondo, J. Antibiot. 1988, 41, 713–718.
- 8β-Amino acids are often-encountered motifs; see:
- 8aP. Spiteller, F. von Nussbaum, β -Amino Acids in Natural Products in Enantioselective Synthesis of β-Amino Acids (Eds.: ), 2nd ed., Wiley, New Jersey, 2005, pp. 19–91;
- 8bF. von Nussbaum, P. Spiteller, β-Amino Acids in Nature in Highlights in Bioorganic Chemistry—Methods and Applications (Eds.: ), Wiley-VCH, Weinheim, 2004, pp. 63–89.
- 9The total synthesis of the ramoplanin aglycon is a recent milestone; see: D. Shin, Y. Rew, D. L. Boger, Proc. Natl. Acad. Sci. USA 2004, 101, 11977–11979.
- 10
- 10aC. Palomo, M. Oiarbide, I. Ganboa, J. I. Miranda, Tetrahedron Lett. 2001, 42, 8955–8957;
- 10bS. Armaroli, G. Cardillo, L. Gentilucci, M. Gianotti, A. Tolomelli, Org. Lett. 2000, 2, 1105–1107;
- 10cG. Cardillo, S. Fabbroni, L. Gentilucci, R. Perciaccante, A. Tolomelli, Komppa Centenary Symposium—100 Years of Natural Product Synthesis, Helsinki, Finland, 2003, Poster ORGN843;
- 10dA. M. Hafez, T. Dudding, T. R. Wagerle, M. H. Shah, A. E. Taggi, T. Lectka, J. Org. Chem. 2003, 68, 5819–5825;
- 10eA. Guzman-Martinez, R. B. Lamer, M. S. VanNieuwenhze, 232th ACS National Meeting, San Francisco, USA, September 10–14, 2006.
- 11A solid-phase synthesis of a close congener of 1 has been described. To reduce the synthetic complexity, HyAsn10 was omitted and substituted by Asn10. Furthermore, HyLeu4 and aThr8 were both exchanged by Thr (no biological data); see: B. J. Egner, M. Bradley, Tetrahedron 1997, 53, 14021–14030.
- 12Y. N. Belokon, K. A. Kochetkov, N. S. Ikonnikov, T. V. Strelkova, S. R. Harutyunyan, A. S. Saghiyan, Tetrahedron: Asymmetry 2001, 12, 481–485.
- 13D. L. Boger, R. J. Lee, P.-Y. Bounaud, P. Meier, J. Org. Chem. 2000, 65, 6770–6772.
- 14
- 14aH. Maki, K. Miura, Y. Yamano, Antimicrob. Agents Chemother. 2001, 45, 1823–1827; for an excellent review on lipid II, see:
- 14bE. Breukink, B. de Kruijff, Nat. Rev. Drug Discovery 2006, 5, 321–332.
- 15
- 15aA. A. Tymiak, D. R. Kirsch, J. O'Sullivan, J. E. McCullough (E. R. Squibb & Sons, Inc.), US 4754018, 1988 [ Chem. Abstr. 1990, 112, 75294];
- 15bF. von Nussbaum, N. Brunner, S. Anlauf, R. Endermann, C. Fürstner, E. Hartmann, J. Köbberling, J. Ragot, G. Schiffer, J. Schuhmacher, N. Svenstrup, J. Telser, M.-A. Brüning (Bayer HealthCare AG), WO 04099239, 2004 [ Chem. Abstr. 2004, 141, 423388].
- 16A. A. Tymiak, T. J. McCormick, S. E. Unger, J. Org. Chem. 1989, 54, 1149–1157.
- 17K. C. Nicolaou, S. A. Snyder, Angew. Chem. 2005, 117, 1036–1069;
10.1002/ange.200460864 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1012–1044.
- 18CCDC-623590 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19J. Blankenstein, J. Zhu, Eur. J. Org. Chem. 2005, 1949–1964.
- 20
- 20aC. T. Walsh, Science 2004, 303, 1805–1810;
- 20bH. Chen, M. G. Thomas, S. E. O'Connor, B. K. Hubbard, M. D. Burkart, C. T. Walsh, Biochemistry 2001, 40, 11651–11659.
- 21Compound 2 was obtained from 1 under buffered conditions: Boc2O, tBuOH/H2O, DIPEA, RT, 44 %.
- 22
- 22aG. Cardillo, L. Gentilucci, A. Tolomelli, C. Tomasini, Synlett 1999, 1727–1730;
- 22bP. G. Mattingly, M. J. Miller, J. Org. Chem. 1983, 48, 3556–3559.