The Crucial Role of the f Electrons in the Bent or Linear Configuration of Uranium Cyanido Metallocenes†
We thank the Direction de l'Energie Nucléaire of the Commissariat à l'Energie Atomique and the Nuclear Fission Safety Program of the European Union under the EUROPART contract (FI6W-CT-2003-508854) for their financial support, the CalMIP for computational time, and Dr. D. Guillaumont for fruitful discussion.
Graphical Abstract
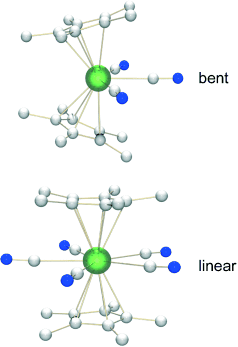
What makes it go linear? The synthesis of the first polycyanide compounds of a 5f element (see picture, U green, N blue, C white) supports the idea that the geometry of metallocenes depends on both the metal oxidation state and the size and shape of the equatorial ligands. Theoretical investigations reveal that the stability of these novel linear π-sandwich compounds is determined by the availability of f orbitals.