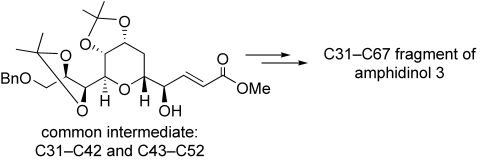
Synthesis of the C31–C67 Fragment of Amphidinol 3†
Javier de Vicente Dr.
Department of Chemistry, University of California, Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-6379
Search for more papers by this authorJohn R. Huckins
Department of Chemistry, University of California, Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-6379
Search for more papers by this authorScott D. Rychnovsky Prof.
Department of Chemistry, University of California, Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-6379
Search for more papers by this authorJavier de Vicente Dr.
Department of Chemistry, University of California, Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-6379
Search for more papers by this authorJohn R. Huckins
Department of Chemistry, University of California, Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-6379
Search for more papers by this authorScott D. Rychnovsky Prof.
Department of Chemistry, University of California, Irvine, 1102 Natural Sciences 2, Irvine, CA 92697-2025, USA, Fax: (+1) 949-824-6379
Search for more papers by this authorThis study was supported by the National Institutes of General Medicine (GM 43854). J.d.V. acknowledges the Spanish Ministry of Education and Science for a postdoctoral fellowship.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z602742_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. K. Paul, N. Matsumori, M. Murata, K. Tachibana, Tetrahedron Lett. 1995, 36, 6279;
- 1bM. Satake, M. Murata, T. Yasumoto, T. Fujita, H. Naoki, J. Am. Chem. Soc. 1991, 113, 9859;
- 1cT. Houdai, S. Matsuoka, M. Murata, M. Satake, S. Ota, Y. Oshima, L. L. Rhodes, Tetrahedron 2001, 57, 5551;
- 1dM. Murata, S. Matsuoka, N. Matsumori, G. K. Paul, K. Tachibana, J. Am. Chem. Soc. 1999, 121, 870;
- 1eG. K. Paul, N. Matsumori, K. Konoki, M. Murata, K. Tachibana, J. Mar. Biotech. 1997, 5, 124;
- 1fG. K. Paul, N. Matsumori, K. Konoki, M. Sasaki, M. Murata, K. Tachibana in Harmful and Toxic Algal Blooms. Proceedings of Seventh International Conference on Toxic Phytoplankton (Eds.: ), UNESCO, Sendai, Japan, 1996, p. 503.
- 2N. Matsumori, D. Kaneno, M. Murata, H. Nakamura, K. Tachibana, J. Org. Chem. 1999, 64, 866.
- 3
- 3aJ. de Vicente, B. Betzemeier, S. D. Rychnovsky, Org. Lett. 2005, 7, 1853;
- 3bS. BouzBouz, J. Cossy, Org. Lett. 2001, 3, 1451;
- 3cE. M. Flamme, W. R. Roush, Org. Lett. 2005, 7, 1411;
- 3dJ. D. Hicks, E. M. Flamme, W. R. Roush, Org. Lett. 2005, 7, 5509;
- 3eL. A. Paquette, S.-K. Chang, Org. Lett. 2005, 7, 3111;
- 3fS.-K. Chang, L. A. Paquette, Synlett 2005, 2915;
- 3gC. Dubost, I. E. Marko, J. Bryans, Tetrahedron Lett. 2005, 46, 4005.
- 4
- 4aL. Ayala, C. G. Lucero, J. A. C. Romero, S. A. Tobacco, K. A. Woerpel, J. Am. Chem. Soc. 2003, 125, 15521;
- 4bR. V. Stevens, A. W. M. Lee, J. Am. Chem. Soc. 1979, 101, 7032;
- 4cR. V. Stevens, Acc. Chem. Res. 1984, 17, 289;
- 4dP. Deslongchamps, Stereoelectronic Effects in Organic Chemistry, Pergamon, New York, 1983, pp. 209–221.
- 5J.-M. Brunel, P. Diter, M. Deutsch, H. B. Kagan, J. Org. Chem. 1995, 60, 8086.
- 6B. M. Trost, S. Mallart, Tetrahedron Lett. 1993, 34, 8025.
- 7The major isomer of 7 was purified by MPLC before the Mosher analysis:
- 7aM. J. Rieser, Y. H. Hui, J. K. Rupprecht, J. F. Kozlowski, K. V. Wood, J. L. McLaughlin, P. R. Hanson, Z. Zhuang, T. R. Hoye, J. Am. Chem. Soc. 1992, 114, 10203;
- 7bJ. A. Dale, H. S. Mosher, J. Am. Chem. Soc. 1973, 95, 512;
- 7cG. R. Sullivan, J. A. Dale, H. S. Mosher, J. Org. Chem. 1973, 38, 2143;
- 7dI. Ohtani, T. Kusumi, Y. Kashman, H. Kakisawa, J. Am. Chem. Soc. 1991, 113, 4092.
- 8
- 8aA. Basha, M. Lipton, S. M. Weinreb, Tetrahedron Lett. 1977, 18, 4171;
- 8bJ. I. Levin, E. Turos, S. M. Weinreb, Synth. Commun. 1982, 12, 989.
- 9D. L. Comins, A. Dehghani, Tetrahedron Lett. 1992, 33, 6299.
- 10W. J. Scott, J. K. Stille, J. Am. Chem. Soc. 1986, 108, 3033.
- 11R. Pappo, D. S. Allen, Jr.,R. U. Lemieux, W. S. Johnson, J. Org. Chem. 1956, 21, 478.
- 12D. B. Dess, J. C. Martin, J. Org. Chem. 1983, 48, 4155.
- 13
- 13aK. Sonagashira, Y. Todah, N. Hagihara, Tetrahedron Lett. 1975, 16, 4467;
- 13bM. Alami, F. Ferri, G. Linstrumelle, Tetrahedron Lett. 1993, 34, 6403.
- 14
- 14aH. C. Brown, S. K. Gupta, J. Am. Chem. Soc. 1972, 94, 4370;
- 14bH. C. Brown, T. Hamaoka, N. Ravindran, J. Am. Chem. Soc. 1973, 95, 5786.
- 15
- 15aE.-I. Negishi, Acc. Chem. Res. 1982, 15, 340;
- 15bE.-I. Negishi, T. Takahashi, S. Baba, D. E. Van Horn, N. Okukado, J. Am. Chem. Soc. 1987, 109, 2393.
- 16
- 16aS. L. Huang, K. Omura, D. Swern, J. Org. Chem. 1976, 41, 3329;
- 16bT. T. Tidwell, Org. React. 1990, 39, 297.
- 17Y. Wang, F. G. West, Synthesis 2002, 99.
- 18LiDBB is a reducing agent for arene radical anions, first reported by Freeman et al.: P. K. Freeman, L. L. Hutchinson, J. Org. Chem. 1980, 45, 1924.
- 19O. Mitsunobu, Synthesis 1981, 1.
- 20H. S. Schultz, H. B. Freyermuth, S. R. Buc, J. Org. Chem. 1963, 28, 1140.
- 21J. R. Parikh, W. v. E. Doering, J. Am. Chem. Soc. 1967, 89, 5505.
- 22
- 22aP. R. Blakemore, W. J. Cole, P. J. Kocienski, A. Morley, Synlett 1998, 26;
- 22bP. R. Blakemore, J. Chem. Soc. Perkin Trans. 1 2002, 2563.
- 23M. Julia, J. M. Paris, Tetrahedron Lett. 1973, 14, 4833.