Dehydroiodination of Iodo- and Diiodomethane by a Transient Phosphinidene Complex†
Aysel Özbolat Dipl.-Chem.
Institut für Anorganische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany, Fax: (+49) 228-739-616 http://www.ak-streubel.uni-bonn.de
Search for more papers by this authorArif Ali Khan Dr.
University School of Basic & Applied Sciences, G.G.S. Indraprastha University, Kashmere Gate, Delhi-110006, India
Search for more papers by this authorGerd von Frantzius Mag.-Chem.
Institut für Anorganische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany, Fax: (+49) 228-739-616 http://www.ak-streubel.uni-bonn.de
Search for more papers by this authorMartin Nieger Dr.
Laboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, P.O. Box 55, 00014 Helsinki, Finland
Search for more papers by this authorRainer Streubel Prof. Dr.
Institut für Anorganische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany, Fax: (+49) 228-739-616 http://www.ak-streubel.uni-bonn.de
Search for more papers by this authorAysel Özbolat Dipl.-Chem.
Institut für Anorganische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany, Fax: (+49) 228-739-616 http://www.ak-streubel.uni-bonn.de
Search for more papers by this authorArif Ali Khan Dr.
University School of Basic & Applied Sciences, G.G.S. Indraprastha University, Kashmere Gate, Delhi-110006, India
Search for more papers by this authorGerd von Frantzius Mag.-Chem.
Institut für Anorganische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany, Fax: (+49) 228-739-616 http://www.ak-streubel.uni-bonn.de
Search for more papers by this authorMartin Nieger Dr.
Laboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, P.O. Box 55, 00014 Helsinki, Finland
Search for more papers by this authorRainer Streubel Prof. Dr.
Institut für Anorganische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany, Fax: (+49) 228-739-616 http://www.ak-streubel.uni-bonn.de
Search for more papers by this authorFinancial support by the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the Ministry of Culture and Education of Lower Saxony (postdoctoral grant for A.A.K.) is gratefully acknowledged. Furthermore, we thank the John von Neumann Institute for Computing (Jülich) for computing time.
Graphical Abstract
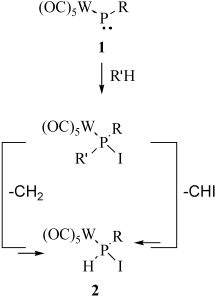
Elimination round: Dehydroiodination occurs when transient phosphinidene complex 1 is treated with iodomethane and diiodomethane, thus formally eliminating CH2 and CHI and giving in both cases complex 2 (see scheme; R=CH(SiMe3)2; R′=CH3, CH2I). The overall reactions represent examples of unprecedented PC bond-cleavage reactions, and proceed under unusually mild conditions.
References
- 1
- 1aV. Plack, J. Goerlich, R. Schmutzler, Z. Anorg. Allg. Chem. 1998, 624, 1940–1942, and references therein;
10.1002/(SICI)1521-3749(1998120)624:12<1940::AID-ZAAC1940>3.0.CO;2-O CAS Web of Science® Google Scholar
- 1bR. Quesada, J. Ruiz, V. Riera, S. Garcia-Granda, M. R. Diaz, Chem. Commun. 2003, 1942–1943;
- 1cM. E. Garcia, V. Riera, M. A. Ruiz, D. Sáez, H. Hamidov, J. C. Jeffery, T. Riis-Johannessen, J. Am. Chem. Soc. 2003, 125, 13044–13045;
- 1dK. Knabel, T. M. Klapötke, H. Nöth, R. T. Paine, I. Schwab, Eur. J. Inorg. Chem. 2005, 1099–1108.
- 2H. Trauner, E. de la Cuesta, A. Marinetti, F. Mathey, Bull. Soc. Chim. Fr. 1995, 132, 384–393.
- 3I. Kolodiazhni, Phosphorus Ylides, Wiley-VCH, Weinheim, 1999.
10.1002/9783527613908 Google Scholar
- 4“1λ5-Phosphinines”: R. Streubel in Science of Synthesis, Vol. 15, Thieme, New York, 2005, pp. 1157–1179.
- 5D. E. C. Corbridge, Phosphorus, 4th ed., Elsevier, Amsterdam, 1990, p. 320.
- 6A. A. Khan, C. Wismach, P. G. Jones, R. Streubel, Dalton Trans. 2003, 2483–2487.
- 7A. A. Khan, C. Wismach, P. G. Jones, R. Streubel, Chem. Commun. 2003, 2892–2893.
- 8R. Streubel, A. Ostrowski, S. Priemer, U. Rohde, J. Jeske, P. G. Jones, Eur. J. Inorg. Chem. 1998, 257–261.
10.1002/(SICI)1099-0682(199802)1998:2<257::AID-EJIC257>3.0.CO;2-H CAS Web of Science® Google Scholar
- 9For an example of a surprising “loss” of CH2 from a P ligand upon coordination to a tungsten center, see: E. Bannwart, H. Jacobsen, R. Hübener, H. W. Schmalle, H. Berke, J. Organomet. Chem. 2001, 622, 97–111.
- 10S. J. Goede, F. Bickelhaupt, Chem. Ber. 1991, 124, 2677–2684.
- 11E. Ionescu, G. von Frantzius, P. G. Jones, R. Streubel, Organometallics 2005, 24, 2237–2240.
- 12R. Appel, C. Casser, F. Knoch, J. Organomet. Chem. 1985, 293, 213–217.
- 13A. H. Cowley, J. E. Kilduff, S. K. Mehrotra, N. C. Norman, M. Pakulski, J. Chem. Soc. Chem. Commun. 1983, 528–529.
- 14Selected NMR (CDCl3, 25 °C; ext. TMS (1H, 13C) or ext. 85 % H3PO4 (31P)) and MS data of complexes 3–5: 3: 1H NMR (200.0 MHz): δ=0.31 (s, 9 H, SiCH3), 0.34 (s, 9 H, SiMe3), 1.85 (d, 2J(P,H)=3.6 Hz, 1 H, CHSiMe3), 3.02 ppm (d, 2J(P,H)=3.5 Hz, 3 H, PCH3); 13C{1H} NMR (50.3 MHz): δ=3.1 (d, 3J(P,C)=3.7 Hz, SiCH3), 3.6 (d, 3J(P,C)=3.0 Hz, SiCH3), 29.2 (d, 1J(P,C)=20.2 Hz, PCH3), 36.1 (d, 1J(P,C)=14.5 Hz, PCH), 198.6 (d, 2J(P,C)=7.8 Hz, cis-CO), 199.7 ppm (d, 2J(P,C)=22.9 Hz, trans-CO); 31P{1H} NMR (81.0 MHz): δ=29.7 ppm (s, 1J(W,P)=263.1 Hz); MS (70 eV, EI, 184W, 127I): m/z (%): 656 (10) [M+]. 4: 1H NMR (200.0 MHz): δ=0.29 (s, 9 H, SiMe3), 0.30 (s, 9 H, SiMe3), 1.35 (d, 2J(P,H)=5.0 Hz, 1 H, PCH), 6.27 ppm (d, 1J(P,H)=337.0 Hz); 13C{1H} NMR (50.3 MHz): δ=0.1 (d, 3J(P,C)=2.8 Hz, SiCH3), 1.3 (d, 3J(P,C)=4.5 Hz, SiCH3), 18.9 (d, 1J(P,C)=12.0 Hz, PCH), 197.1 (d, 2J(P,C)=6.7 Hz, cis-CO), 198.3 ppm (d, 2J(P,C)=22.1 Hz, trans-CO); 31P{1H} NMR (81.0 MHz): δ=−49.4 (ddsat, 1J(P,H)=337.0 Hz, 1J(W,P)=254.0, 2J(P,H)=4.2 Hz); MS (70 eV, EI, 184W,127I): m/z (%): 641 (4) [M+]. 5: 1H NMR (300.0 MHz): δ=0.42 (s, 18 H, SiMe3), 1.27 (d, 1 H, PCH), 2.11 ppm (d, 2J(P,H)=12.7 Hz, 9 H, PCH3); 13C{1H} NMR (75.5 MHz): δ=2.1 (d, 3J(P,C)=2.9 Hz, SiMe3), 9.8 (d, 1J(P,C)=26.5 Hz, PCH), 14.2 ppm (d, 1J(P,C)=55.3 Hz, PMe3); even at −50 °C the CO resonance was not observed; 31P{1H} NMR (121.5 MHz): δ=26.0 (mc, 2J(P,H)=12.7 Hz).
- 15Crystal structure data for complex 5 (C14H28I3O4PSi2W): orthorhombic, space group Pbca (no. 61), a=17.5907(2), b=17.4856(2), c=34.6938(4) Å, V=10 671.3(2) Å3, Z=16, ρ=2.271 Mg m−3, μ(MoKα)=7.966 mm−1. A total of 47 812 reflections were measured on a Nonius KappaCCD diffractometer using MoKα radiation (λ=0.71073 Å) at a temperature of 123(2) K, 9412 reflections were unique (Rint=0.0697). A semiempirical absorption correction from equivalents was applied (min./max. transmission=0.21995/0.41737). The structure was solved with Patterson methods and refined with full-matrix least squares against F2 of all reflections. Non-hydrogen atoms were refined anisotropically, hydrogen atoms were refined as rigid groups. R values [I>2σ(I)]: R1=0.0263, wR2=0.0432. R values for all data: R1=0.0457, wR2=0.0462, min./max. difference electron density −0.918/0.750 e Å−3. CCDC-606431 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 16N. Kuhn, R. Jüschke, W.-W. du Mont, M. Bätcher, D. Bläser, R. Boese, Z. Naturforsch. B 1989, 44, 9–12.
- 17W. Malisch, Angew. Chem. 1973, 85, 228; Angew. Chem. Int. Ed. Engl. 1973, 12, 235.
- 18Gaussian 03, RevB.02, 2003, Gaussian Inc. B3LYP: A. D. Becke, J. Chem. Phys. 1993, 98, 5648. Valence triple zeta + polarization basis 6-311g(d,p): R. Krishnan, J. S. Binkley, R. Seeger, J. A. Pople, J. Chem. Phys. 1980, 72, 650; A. D. McLean, G. S. Chandler, J. Chem. Phys. 1980, 72, 5639. For iodine the 6-311g(d,p) basis set from the EMSL Gaussian basis set order form (www.emsl.pnl.gov/forms/basisform.html) was used. ECP LanL2DZ at W: P. J. Hay, W. R. Wadt, J. Chem. Phys. 1985, 82, 270.
- 19Compliance constants (diagonal elements of the inverse of the matrix of force constants) are inversely proportional to the strength of a bond; the smaller the numerical value the less compliant it is. J. C. Decius, J. Chem. Phys. 1963, 38, 241; L. H. Jones, B. I. Swanson, Acc. Chem. Res. 1976, 9, 128.
- 20F. A. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, R. Taylor, J. Chem. Soc. Perkin Trans. 2 1987, S1.
- 21Y. V. Zefirov, Russ. J. Inorg. Chem. 2001, 46, 568–572.
- 22
- 22aJ. G. Leipoldt, E. C. Steynberg, R. van Eldik, Inorg. Chem. 1987, 26, 3068–3070;
- 22bF. Morandini, G. Consiglio, J. Chem. Soc. Chem. Commun. 1991, 676–677.
- 23H. E. Simmons, R. D. Smith, J. Am. Chem. Soc. 1958, 80, 5323–5324.
- 24Recently, the synthesis of alkylidine (from alkylidene) complexes was achieved by dehydrohalogenation by a germylene derivative: S. R. Caskey, M. H. Stewart, Y. J. Ahn, M. J. A. Johnson, J. W. Kampf, Organometallics 2005, 24, 6074–6076.