Enantioselective Total Synthesis of (−)-Acylfulvene and (−)-Irofulven†
Mohammad Movassaghi Prof. Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Search for more papers by this authorGrazia Piizzi Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Search for more papers by this authorDustin S. Siegel
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Search for more papers by this authorGiovanni Piersanti Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Current address: Universitá degli Studi di Urbino “Carlo Bo”, Istituto di Chimica Farmaceutica, Piazza Rinascimento 6, 61029 Urbino, Italy
Search for more papers by this authorMohammad Movassaghi Prof. Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Search for more papers by this authorGrazia Piizzi Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Search for more papers by this authorDustin S. Siegel
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Search for more papers by this authorGiovanni Piersanti Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-254-1504 http://web.mit.edu/movassag/www/index.htm
Current address: Universitá degli Studi di Urbino “Carlo Bo”, Istituto di Chimica Farmaceutica, Piazza Rinascimento 6, 61029 Urbino, Italy
Search for more papers by this authorM.M. is a Dale F. and Betty Ann Frey Damon Runyon Scholar supported by the Damon Runyon Cancer Research Foundation (DRS-39-04). M.M. is a Firmenich Assistant Professor of Chemistry. G.P. acknowledges partial support for a postdoctoral fellowship from Universitá degli Studi di Urbino “Carlo Bo”. We are grateful to Professors A. H. Hoveyda and E. N. Jacobsen for generous samples of their silylcyanation catalysts. We acknowledge financial support by MIT, Paul M. Cook Fund, and Boehringer Ingelheim Pharmaceutical Inc.
Graphical Abstract
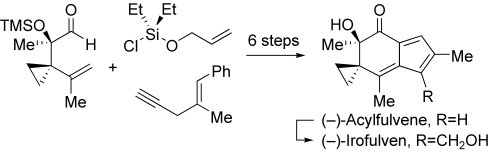
Antitumor agents (−)-acylfulvene and (−)-irofulven are prepared in an approach that employs the powerful enyne ring-closing metathesis reaction to secure the spiro-bicyclic AB rings. Other key features of this synthesis include an efficient aldol-based introduction of the stereocenter at C2, a diazene-mediated reductive allylic transposition, and a ring-closing metathesis/oxidation sequence.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z602011_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Anchel, A. Hervey, W. J. Robbins, Proc. Natl. Acad. Sci. USA 1952, 38, 927;
- 1bT. C. McMorris, M. J. Kelner, W. Wang, J. Yu, L. A. Estes, R. Taetle, J. Nat. Prod. 1996, 59, 896.
- 2
- 2aJ. Alexandre, E. Raymond, M. O. Kaci, E. C. Brian, F. Lokiec, C. Kahatt, S. Faivre, A. Yovine, F. Golwasser, S. L. Smith, J. R. MacDonald, J.-L. Misset, E. Cvitkovic, Clin. Cancer Res. 2004, 10, 3377;
- 2bJ. Gong, J. F. Neels, X. Yu, T. W. Kensler, L. A. Peterson, S. J. Sturla, J. Med. Chem. 2006, 49, 2593;
- 2cM. Serova, F. Calvo, F. Lokiec, F. Koeppel, V. Poindessous, A. K. Larsen, E. S. Van Laar, S. J. Waters, E. Cvitkovic, E. Raymond, Cancer Chemother. Pharmacol. 2006, 57, 491;
- 2dhttp://cancernet.nci.nih.gov/clinicaltrials.
- 3Enantioselective synthesis of (−)-4:
- 3aT. C. McMorris, M. D. Staake, M. J. Kelner, J. Org. Chem. 2004, 69, 619; formal enantioselective synthesis of (−)-4:
- 3bK. M. Brummond, J. Lu, J. Peterson, J. Am. Chem. Soc. 2000, 122, 4915; also, see: K. M. Brummond, J. Lu, J. Am. Chem. Soc. 1999, 121, 5087.
- 4For recent reviews, see:
- 4aK. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. 2005, 117, 4564;
10.1002/ange.200500369 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4490; ;
- 4bS. T. Diver, A. Giessert, Chem. Rev. 2004, 104, 1317; for representative examples, see:
- 4cS.-H. Kim, N. Bowden, R. H. Grubbs, J. Am. Chem. Soc. 1994, 116, 10801;
- 4dW. J. Zuercher, M. Scholl, R. H. Grubbs, J. Org. Chem. 1998, 63, 4291;
- 4eM. E. Layton, C. A. Morales, M. D. Shair, J. Am. Chem. Soc. 2002, 124, 773;
- 4fH. Fukumoto, K. Takanishi, J. Ishihara, S. Hatakeyama, Angew. Chem. 2006, 118, 2797;
10.1002/ange.200600210 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2731.
- 5
- 5aR. H. Grubbs, Tetrahedron 2004, 60, 7117;
- 5bR. R. Schrock, A. H. Hoveyda, Angew. Chem. 2003, 115, 4740;
10.1002/ange.200300576 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4592;
- 5cA. Fürstner, Angew. Chem. 2000, 112, 3140;
10.1002/1521-3757(20000901)112:17<3140::AID-ANGE3140>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3012.10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 6
- 6aD. A. Evans, C. S. Burgey, M. C. Kozlowski, S. W. Treay, J. Am. Chem. Soc. 1999, 121, 686;
- 6bseveral other catalytic asymmetric routes to 11 (namely, cyanation of the corresponding methyl ketone or dihydroxylation of a related diene) were explored and ultimately the most efficient route was selected.
- 7Please see the Supporting Information for details.
- 8
- 8aH. Tokuyama, S. Yokoshima, T. Fukuyama, Tetrahedron Lett. 1998, 39, 3189;
- 8bJ. E. Milne, S. L. Buchwald, J. Am. Chem. Soc. 2004, 126, 13028.
- 9K. Takai, T. Kakiuchi, Y. Kataoka, K. Utimoto, J. Org. Chem. 1994, 59, 2668.
- 10For reports on other natural illudins, see:
- 10aA. Arnone, R. Cardillo, G. Nasini, O. V. De Pava, J. Chem. Soc. Perkin Trans. 1 1991, 733;
- 10bA. Arnone, R. Cardillo, V. di Modugno, G. Nasini, Gazz. Chim. Ital. 1991, 121, 345;
- 10cI.-K. Lee, C.-Y. Jeong, S.-M. Cho, B.-S. Yun, Y.-S. Kim, S.-H. Yu, H. Koshino, I.-D. Yoo, J. Antibiot. 1996, 49, 821;
- 10dC. Dufresne, K. Young, F. Pelaez, A. González del Val, D. Valentino, A. Graham, G. Platas, A. Bernard, D. Zink, J. Nat. Prod. 1997, 60, 188;
- 10eM. L. Burgess, Y. L. Zhang, K. D. Barrow, J. Nat. Prod. 1999, 62, 1542;
- 10fM. Reina, J. C. Orihuela, A. Gonzáles-Coloma, C. de Inés, M. de la Crus, A. González del Val, J. R. Torno, B. M. Fraga, Phytochemistry 2004, 65, 381.
- 11For representative syntheses of cyclohexenes containing a tetrasubstituted olefin through enyne metathesis, see:
- 11aT. Kitamura, Y. Sato, M. Mori, Chem. Commun. 2001, 14, 1258;
- 11bT. Kitamura, Y. Sato, M. Mori, Adv. Synth. Catal. 2002, 344, 678.
- 12The experimental procedure for the preparation of diol 22 is given in the Supporting Information.
- 13M. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953.
- 14For a review on silicon-tether reactions, see:
- 14aL. Festerbank, M. Malacria, S. M. Sieburth, Synthesis 1997, 813; for representative examples of olefin metathesis mediated by an allyldialkylsilicon tether, see:
10.1055/s-1997-1295 Google Scholar
- 14bS. Chang, R. H. Grubbs, Tetrahedron Lett. 1997, 38, 4757;
- 14cQ. Yao, Org. Lett. 2001, 3, 2069.
- 15For other synthetic studies, see:
- 15aT. Matsumoto, H. Shirahama, A. Ichihara, H. Shin, S. Kagawa, F. Sakan, S. Matsumoto, S. Nishida, J. Am. Chem. Soc. 1968, 90, 3280;
- 15bF. R. Kinder, K. W. Bair, J. Org. Chem. 1994, 59, 6965;
- 15cA. Padwa, V. P. Sandanayaka, E. A. Curtis, J. Am. Chem. Soc. 1994, 116, 2667;
- 15dH. Primke, G. S. Sarin, S. Kohlstruk, G. Adiwidjaja, A. de Meijere, Chem. Ber. 1994, 127, 1051;
- 15eT. C. McMorris, J. Yu, Y. Hu, J. Org. Chem. 1997, 62, 3015;
- 15fF. R. Kinder, R. M. Wang, W. E. Bauta, K. W. Bair, Synth. Commun. 1997, 62, 521;
- 15gT. C. McMorris, Q. Cong, M. J. Kelner, J. Org. Chem. 2003, 68, 9648;
- 15hM. C. Pirrung, H. Liu, Org. Lett. 2003, 5, 1983.
- 16K. Tamao, N. Ishida, M. Kumada, Org. Synth. 1990, 69, 96; for examples of a Tamao oxidation of silicon-tethered metathesis products, see Ref. [14b–c].
- 17For examples of metathesis reactions using allyloxysilicon tethers, see:
- 17aT. R. Hoye, M. A. Promo, Tetrahedron Lett. 1999, 40, 1429;
- 17bS. S. Zhu, D. R. Cefalo, D. S. La, J. Y. Jamieson, W. M. Davis, A. H. Hoveyda, R. R. Schrock, J. Am. Chem. Soc. 1999, 121, 8251;
- 17cA. Briot, M. Bujard, V. Gouverneur, S. P. Nolan, C. Mioskowski, Org. Lett. 2000, 2, 1517;
- 17dP. Van de Weghe, D. Aoun, J.-G. Boiteau, J. Eustache, Org. Lett. 2002, 4, 4105;
- 17eM. H. Postema J. L. Piper, Tetrahedron Lett. 2002, 43, 7095;
- 17fP. E. Evans, J. Cui, S. J. Gharpure, A. Polosukhin, H.-R. Zhang, J. Am. Chem. Soc. 2003, 125, 14702.
- 18Initial EYRCM reactions to test the new allyloxydiethylsilyl tether were successfully carried out on dienynes related to those described in Table 1.
- 19The EYRCM reaction of the C2 hydroxy variants of trienynes 23 a–b exclusively gave the desired triols 25 a–b, respectively, in approximately 50 % yield of the isolated product, but required a longer reaction time and higher catalyst loadings.
- 20The trienyne 23 b was prepared according to the procedure described for the trienyne 23 a.
- 21
- 21aA. G. Myers, B. Zheng, Tetrahedron Lett. 1996, 37, 4841;
- 21bA. G. Myers, M. Movassaghi, B. Zheng, J. Am. Chem. Soc. 1997, 119, 8572.