Enantioselective Oxidation of Olefins Catalyzed by a Chiral Bishydroxamic Acid Complex of Molybdenum†
Allan U. Barlan
Department of Chemistry, University of Chicago, 5735 South Ellis Avenue, Chicago, Illinois 60637, USA, Fax: (+1) 773-702-0805
Search for more papers by this authorArindrajit Basak Dr.
Department of Chemistry, University of Chicago, 5735 South Ellis Avenue, Chicago, Illinois 60637, USA, Fax: (+1) 773-702-0805
Search for more papers by this authorHisashi Yamamoto Prof. Dr.
Department of Chemistry, University of Chicago, 5735 South Ellis Avenue, Chicago, Illinois 60637, USA, Fax: (+1) 773-702-0805
Search for more papers by this authorAllan U. Barlan
Department of Chemistry, University of Chicago, 5735 South Ellis Avenue, Chicago, Illinois 60637, USA, Fax: (+1) 773-702-0805
Search for more papers by this authorArindrajit Basak Dr.
Department of Chemistry, University of Chicago, 5735 South Ellis Avenue, Chicago, Illinois 60637, USA, Fax: (+1) 773-702-0805
Search for more papers by this authorHisashi Yamamoto Prof. Dr.
Department of Chemistry, University of Chicago, 5735 South Ellis Avenue, Chicago, Illinois 60637, USA, Fax: (+1) 773-702-0805
Search for more papers by this authorSupport for this research was provided by the SORST project of the Japan Science and Technology Agency (JST) and GAANN.
Graphical Abstract
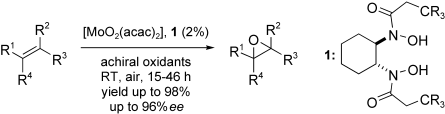
Excellent yields and enantioselectivities can be achieved in the molybdenum–bishydroxamic acid catalyzed asymmetric oxidation of olefins in air at room temperature with an achiral oxidant (see scheme; acac=acetylacetonate). A range of terminal, cis-, and trisubstituted olefins can be used as the substrate. Furthermore, when there are multiple double bonds, the most electron-rich is oxidized.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z601742_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Jorgensen, Chem. Rev. 1989, 89, 431;
- 1bC. Bonini, R. Giuliana, Tetrahedron 2002, 58, 4981;
- 1cT. Katsuki, E. N. Jacobsen, M. H. Wu in Comprehensive Asymmetric Catalysis I–III (Eds.: ), Springer, Berlin, 1999, pp. 621–678;
- 1dI. Ojima, Catal. Asymmetric Synthesis, Wiley-VCH, New York, 2000, pp. 231–325;
10.1002/0471721506 Google Scholar
- 1eH. Adolfsson in Modern Oxidation Methods (Eds.: ), Wiley-VCH, Weinheim, 2004, pp. 21–49;
10.1002/3527603689.ch2 Google Scholar
- 1fT. Katsuki, J. Am. Chem. Soc. 1980, 102, 5974.
- 2
- 2aR. Irie, K. Noda, Y. Ito, N. Matsumoto, T. Katsuki, Tetrahedron Lett. 1990, 31, 7345;
- 2bW. Zhang, J. L. Loebach, S. R. Wilson, E. N. Jacobsen, J. Am. Chem. Soc. 1990, 112, 2801.
- 3
- 3aY. Shi, Acc. Chem. Res. 2004, 37, 3435;
- 3bM. Hickey, D. Goeddel, Z. Crane, Y. Shi, Proc. Natl. Acad. Sci. USA 2004, 101, 5794.
- 4
- 4aY. Hoshino, H. Yamamoto, J. Org. Chem. 1999, 64, 338;
- 4bY. Hoshino, N. Murase, M. Oishi, H. Yamamoto, Bull. Chem. Soc. Jpn. 2000, 73, 1653;
- 4cY. Hoshino, H. Yamamoto, J. Am. Chem. Soc. 2000, 122, 10452.
- 5
- 5aW. Zhang, A. Basak, Y. Kosugi, Y. Hoshino, H. Yamamoto, Angew. Chem. 2005, 117, 4463; Angew. Chem. Int. Ed. 2005, 44, 4389;
- 5bA. Basak, A. Barlan, H. Yamamoto, Tetrahedron: Asymmetry 2006, 17, 508.
- 6The vanadium–2 a complex in the presence of TBHP selectively oxidized geraniol to generate 2,3-epoxygeraniol.
- 7O. Bortolini, S. Campestrini, E. Furia, G. Modena, J. Org. Chem. 1987, 52, 5093.
- 8
- 8aM. N. Sheng, G. J. Zajaczek (ARCO), GB 1.136.923, 1968;
- 8bJ. Kolar (Halcon), US 3.350.422, US 3.351.635, 1967.
- 9V. Schurig, K. Hintzer, U. Leyrer, C. Mark, P. Pitchen, H. Kagan, J. Organomet. Chem. 1989, 370, 81.
- 10
- 10aS. Yamada, T. Mashiko, S. Tershima, J. Am. Chem. Soc. 1977, 99, 1988;
- 10bR. C. Michaelson, R. E. Palermo, K. B. Sharpless, J. Am. Chem. Soc. 1977, 99, 1990;
- 10cF. Kuhn, J. Zhao, W. A. Herrmann, Tetrahedron: Asymmetry 2005, 16, 3469, and references therein.
- 11
- 11aK. Ambroziak, R. Peloch, E. Milchert, T. Dziemboska, Z. Rozwadowski, J. Mol. Catal. A 2004, 211, 9;
- 11bM. Masteri-Farahani, F. Farzaneh, M. Ghandhi, J. Mol. Catal. A 2003, 192, 103;
- 11cS. Kotov, T. Kolev, M. Georgrieva, J. Mol. Catal. A 2003, 195, 83;
- 11dI. Gonclaves, A. Santos, C. Romao, A. Lopes, J. Rodriguez-Borges, M. Pillinger, P. Ferriera, J. Rocha, R. Kuhn, J. Organomet. Chem. 2001, 626, 1;
- 11eX. Wang, H. Shi, C. Sun, Z. Zhang, Tetrahedron 2004, 60, 10993;
- 11fJ. Fridgen, W. A. Herrmann, G. Eickerling, A. Santos, F. Kuhn, J. Organomet. Chem. 2004, 689, 2752.
- 12OSHA recommends exposure to Mo to be 15 mg m−3 versus 5 mg m−3 of Mn.
- 13H. Yale, Chem. Rev. 1943, 43, 209.
10.1021/cr60106a002 Google Scholar
- 14
- 14aS. Raina, V. Singh, Tetrahedron 1995, 51, 2467;
- 14bE. J. Corey, M. Noe, W. Shieh, Tetrahedron Lett. 1993, 34, 5995.
- 15
- 15aD. Deubal, J. Sundermeyer, G. Frenking, J. Am. Chem. Soc. 2000, 122, 10101;
- 15bD. Deubal, J. Sundermeyer, G. Frenking, Eur. J. Inorg. Chem. 2001, 1819;
10.1002/1099-0682(200107)2001:7<1819::AID-EJIC1819>3.0.CO;2-K Google Scholar
- 15cI. Yadanov, C. Valentin, P. Gisdakis, N. Rosch, J. Mol. Catal. A 2000, 158, 189;
- 15dH. Mimoun, Angew. Chem. 1982, 94, 750; Angew. Chem. Int. Ed. Engl. 1982, 21, 734;
- 15eD. Deubal, G. Frenking, P. Gisdakis, W. A. Herrmann, N. Rosch, J. Sundermeyer, Acc. Chem. Res. 2004, 37, 645;
- 15fK. B. Sharpless, J. M. Townsend, D. R. Williams, J. Am. Chem. Soc. 1972, 94, 295.