Improving Quantum Efficiencies of Siloles and Silole-Derived Butadiene Chromophores through Structural Tuning†
We are grateful to the Robert A. Welch Foundation and donors of the American Chemical Society Petroleum Research fund for partial financial support of this research. A.J.B. thanks the Robert A. Welch Foundation for a Welch Summer Research Fellowship. We are indebted to Dr. Vincent Lynch for determination of crystal structures. We thank FMC Lithium, a division of FMC Corporation, for a generous donation of tBu2Si(OTf)2.
Graphical Abstract
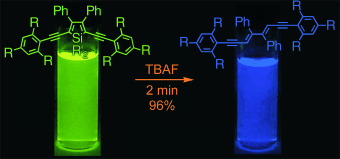
A brighter future for siloles. Luminescent silole chromophores with the highest quantum efficiencies reported for a fully substituted monomeric silole are generated by altering the steric properties of the substituent groups. Treatment of 1,1-dimethylsiloles with Bu4NF (TBAF) in THF rapidly gives stereospecific desilylation at room temperature (see scheme). Intense solid-state photoluminescence is observed in thin films of the silole fluorophores.