Inner Core Structure Responds to Communication between Nanocapsule Walls†
This work was supported by the National Science Foundation.
Graphical Abstract
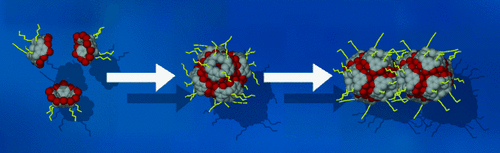
The facile self-assembly of six pyrogallol[4]arene molecules leads to a globular truncated octahedron, which encapsulates a guest cluster consisting of ethyl acetate and water molecules (see scheme). The interactions between host capsules can be controlled by derivatization of the exterior surface of the capsule.
The internal symmetry and resulting packing motifs of microspheres have attracted much attention in recent years.1 Albeit at a higher level of complexity, cell–cell and cell–substrate interactions in biological systems are also under intense investigation.2 For example, the cell wall of the cowpea chlorotic mottle virus undergoes a reversible physiological change whereby it opens and closes on demand to allow passage to and from the interior cavity.3 Supramolecular self-assembled capsules are potentially useful models for such complex biological processes. They are also promising with regard to nanotechnologies involving drug delivery, clean chemical synthesis, assembly of novel materials, and separation science.4–7
Despite keen interest in the self-assembly of simple organic building blocks into molecular containers, there is generally little information on the fate of the encapsulated guest molecules.8–10 Although the confined guest matrix within these molecular containers has been regarded as a new phase of matter,11 a detailed understanding of the interplay and relative orientations of the constituent guest molecules has, until now, been restricted to a few instances of limited complexity.12, 13
We previously described the self-assembly of the pyrogallol[4]arene building block into a globular hexamer, which is stable even in aqueous media.8, 14 To date, only capsules composed of C-isobutylpyrogallol[4]arene8, 14, 15 and C-propylpyrogallol[4]arene16 have been structurally authenticated by X-ray single-crystal structure analysis. In these studies, it was not possible to determine any geometrical information relating to the included guest molecules. Indeed, the exact nature of their relationship with the host and with one another has been a matter for speculation.10, 17 We now show for the first time that the guest molecules trapped within the host container adjust their spatial orientation in response to interactions between adjacent nanocapsules. Remarkably, functionalizing the outer shell of the nanocapsule with different alkyl chains leads to a highly specific solid-state packing arrangement, which in turn influences the organization of the guest molecules within the cavitands.
Pyrogallol[4]arene macrocyclic building blocks 1 a–3 a (Scheme 1) can be readily prepared through “green technologies”, by the solvent-free acid-catalyzed condensation of pyrogallol and an appropriate aldehyde.18 Crystallization of 1 a–3 a from common solvents under atmospheric conditions usually yields the kinetically favored bilayer structure15, 19 in which the lipophilic tails interdigitate with one another and the phenolic groups undergo hydrogen bonding to adjacent molecules to form sheetlike arrays. The protocol that was previously employed to effect the self-assembly of 1 a–3 a into spherical hexamers 1–3 has now been superseded by the simpler method of recrystallizing the compounds from a solution in ethyl acetate. Thus, nanocapsules 1–3 have been synthesized reproducibly in quantitative yields under normal laboratory conditions (Scheme 1).
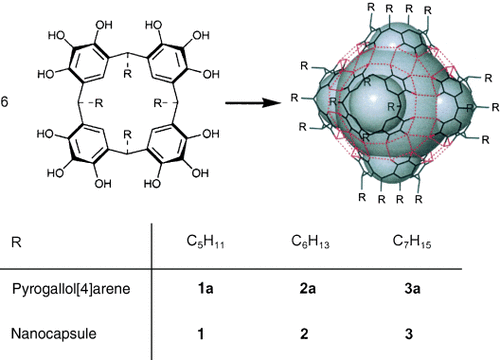
Synthesis and structure of nanocapsules 1–3.
Single-crystal X-ray diffraction analysis of the self-assembled nanocapsules 1–3 reveals that, in each case, six pyrogallol[4]arene macrocycles 1 a–3 a associate into the previously described globular hexamer, with a diameter of approximately 4 nm (Figure 1).8, 14, 16 The assembly is stabilized by a total of 72 hydrogen bonds, of which 48 are intermolecular (eight intermolecular hydrogen bonds per bound macrocycle). In contrast, the self-assembled capsule consisting of six resorcin[4]arene units and eight water molecules in the form of an Archimedean snub cube,20 which is capable of housing up to eight benzene molecules, utilizes only 60 hydrogen bonds, of which 36 are intermolecular (only 2.6 intermolecular hydrogen bonds per bound entity).10, 17 The increased number of hydrogen bonds coupled with the fact that nanocapsules 1–3 each consist of only six building blocks is a factor in stabilizing the nanocapsules in polar media.14 Indeed, this remarkable stability is of great significance for the possible exploitation of such assemblies for biological applications.
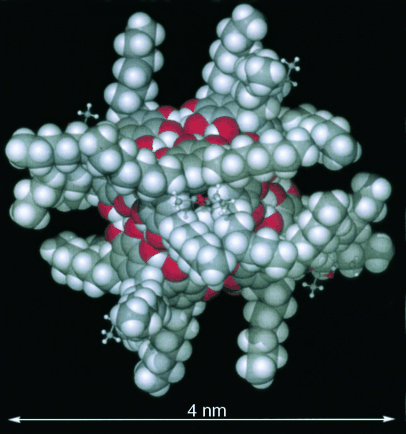
Space-filling view of the molecular structure of nanocapsule 3.
TG–IR (thermogravimetric–infrared) analysis of 3 reveals that ethyl acetate molecules are released from the crystalline material at two distinct stages during gradual heating of a dried sample: A weight loss of 7.5 % (70–105 °C) corresponds to the loss of only the six ethyl acetate molecules external to the cavity; a further weight loss of 8 % immediately prior to decomposition (225–275 °C) corresponds to the loss of both bound ethyl acetate and water molecules. In comparison, TG–IR analysis of monomer 3 a shows no significant weight loss above the boiling point of the cosolvent, until rapid decomposition occurs at 290 °C. Consistent with the TG–IR analysis results, X-ray single-crystal structure analysis of 3 shows that six ethyl acetate molecules enshroud a disordered water molecule within the host assembly. Furthermore, 1H NMR spectroscopy studies of 3 in CDCl3 indicate that there are a total of twelve ethyl acetate molecules per nanocapsule. We infer from the two-step loss of ethyl acetate from the crystalline material and the X-ray crystal-structure analysis of nanocapsule 3 that the ethyl acetate molecules positioned outside the constraints of the nanocapsule are freed between 70 and 105 °C from the pockets traversing the lattice. The guest matrix of ethyl acetate and water is held within the crystal lattice by the nanocapsules until they rupture at approximately 225 °C. The molecular formula is therefore [(C-heptylpyrogallol[4]arene)6n(EtOAc)6(water)]⋅6(EtOAc), with a molecular weight of 6746.72. The initial 7.5 % weight loss is thus consistent with the loss of six ethyl acetate molecules situated in the lattice and exterior to the capsules. The subsequent 8 % weight loss is consistent with the simultaneous loss of one water molecule and six ethyl acetate molecules.
In all cases the nanocapsules contain seven guest molecules (six ethyl acetate molecules and one water molecule). The number of external solvating ethyl acetate molecules depends on the length of the alkyl tail unit. X-ray single-crystal structure analysis reveals that nanocapsule 1 traps six ethyl acetate molecules within pockets formed between alkyl chains: three ethyl acetate molecules reside at the base of three of the macrocyclic building blocks 1 a and three are trapped within the space formed by the interdigitation of adjacent nanocapsule tail groups. Eight ethyl acetate molecules external to nanocapsule 2 are observed by X-ray crystal-structure analysis: two are found at the base of two monomers 2 a and six are trapped within neighboring nanocapsule tail groups. The X-ray single-crystal structure analysis of nanocapsule 3 shows that the six ethyl acetate molecules outside the nanocapsule are all embedded within the alkyl chains at the base of the each of the macrocyclic monomers 3 a.
Nanocapsules 1 and 3 arrange into simple hexagonal and hexagonal-closest-packing arrays (Figure 2 a and b, respectively). The nanocapsules are separated by their lipophilic tails. Notably, the alkyl chains of different capsules only partially interpenetrate as they form lipophilic membranes between the nanocapsules. Remarkably, when the lipophilic tail is six carbon atoms in length the nanocapsules are not forced away from one another, but rather congregate to form hydrogen-bonded nanorods(2, Figure 2 c, d, and Figure 3. Four OH groups on opposite sides of each capsule form hydrogen bonds with two adjacent capsules (O⋅⋅⋅O=2.732, 3.146, and 3.174 Å, Figure 3). Consequently, the walls of the individual nanocapsules are disrupted, which translates through to the packing of the guest matrix. The distorted intermolecular hydrogen bonding within the host walls enables four of the ethyl acetate guest molecules to undergo hydrogen bonding to the nanocapsule wall through their carbonyl functionalities (O⋅⋅⋅O=2.763 and 2.848 Å, Figure 3).
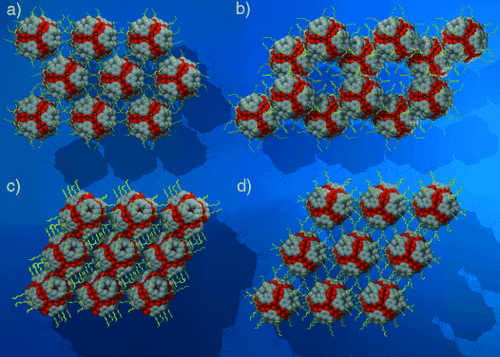
Molecular packing diagram of nanocapsules a) 1, b) 3, and c), d) 2.
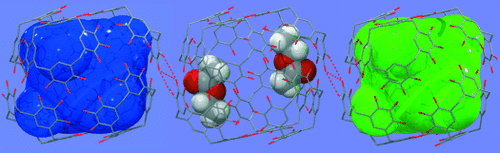
Hydrogen-bonding interactions in nanocapsules 2 and between adjoining nanocapsules.
Nanocapsules 1 and 3 feature ordered arrays of ethyl acetate molecules enshrouding an isolated water molecule. The methyl groups are orientated towards the base of the pyrogallol[4]arene macrocycles and the single water molecule resides at the center of the matrix (Figure 4 a). When the neighboring nanocapsules communicate with each other through the hydrogen-bonding interactions observed in nanocapsule 2, the four ethyl acetate guest molecules hydrogen bonded to the inside of the nanocapsule wall reorient with their ethyl groups in the base of macrocycle (Figure 4 b). The two remaining ethyl acetate guests position their carbonyl groups down into the cleft of the macrocycle, as observed in 1 and 3. It was not possible to locate the exact position of the water molecule inside this nanocapsule owing to extensive disorder. This difference in guest interactions and hence fluidity is clearly a consequence of communication or lack thereof between the host walls of neighboring nanocapsules.
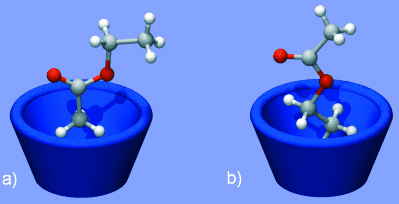
Ethyl acetate orientation within the nanocapsule: a) methyl group down, b) ethyl group down.
The internal volumes21 of 1 and 3 are ≈1300 Å3, whereas 2 encloses only ≈1200 Å3. The smaller volume of 2 is attributed to interactions between neighboring capsules, whereby one of the pyrogallol units within the aromatic plane formed between three hydrogen-bonding pyrogallol[4]arene building blocks also participates in hydrogen-bonding interactions with an adjoining nanocapsule. Consequently the symmetry of the nanocapsule is distorted as the pyrogallol[4]arene building blocks twist to accommodate the intercapsule hydrogen bonding. This disruption of the hexagonal plane formed between the three monomers causes a deviation from planarity of approximately 46° (O211 O111 C143) and is unique to nanocapsule 2.
The guest molecules occupy 40 % of the total volume of the larger cavitands and 44 % of that of distorted conformer 2.21 Although this is significantly lower than the optimal 55 % packing efficiency,22 it is in excellent agreement with the ∼43 % occupancy recently calculated for the eight benzene guest molecules observed within the resorcin[4]arene/water Archimedean snub cube.10
We have shown that a small structural variation—in this case the extension of an alkyl chain by one carbon atom—in the pyrogallol[4]arenes can have profound effects on the crystal packing and interactions between nanocapsules. Communication between the nanocapsules is then translated though the capsule walls to the guest matrix trapped within. The geometry of the guests within nanocapsules 1–3 provides valuable insight into the interactions and fluidity of the so-called “new phase of matter”.11
Experimental Section
The pyrogallol[4]arenes were synthesized through a modified solvent-free technique analogous to that used in the synthesis of resorcin[4]arenes.18 The nanocapsules were synthesized as a crystalline material (suitable for analysis by X-ray diffraction techniques) by slow evaporation of ethyl acetate (≈5 mL) from the pyrogallol[4]arene (≈150 mg).
Typically, thermogravimetric analysis was performed on a dry 18.464-mg crystalline sample of the nanocapsule on a TS Q50 Thermogravimetric Analyzer coupled to a Thermo Nicolet AEM FTIR with a Nexus TGA–IR interface. The sample was heated at a constant rate of 1 °C min−1 from 30 to 500 °C and purged with a continuous stream of nitrogen gas at a flow rate of 20 mL min−1. The IR spectra of the mobile phase in the range of 1000 to 3000 cm−1 were collected at a rate of one spectrum min−1.
Crystal data can be found in the Supporting information. CCDC-228 139 (capsule 1), CCDC-228 138 (capsule 2), and CCDC-228 137 (capsule 3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44)1223–336–033; or [email protected]).