An Amphotericin B–Fluorescein Conjugate as a Powerful Probe for Biochemical Studies of the Membrane†
Andreas Zumbuehl
Laboratorium für Organische Chemie, ETH Hönggerberg, HCI H335, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorDamien Jeannerat Dr.
Départment de Chimie Organique, Université de Genève, 1211 Genève 4, Switzerland
Search for more papers by this authorScott E. Martin Dr.
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorMarc Sohrmann Dr.
Institut für Biochemie, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorPasquale Stano
Department of Chemistry, The Pennsylvania State University, Pennsylvania 16802, USA
Search for more papers by this authorTamas Vigassy Dr.
Laboratorium für Organische Chemie, ETH-Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorDaniel D. Clark
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorStephen L. Hussey Dr.
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorMathias Peter Prof. Dr.
Institut für Biochemie, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorBlake R. Peterson Prof. Dr.
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorErnö Pretsch Prof. Dr.
Laboratorium für Organische Chemie, ETH-Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorPeter Walde Prof. Dr.
Department of Chemistry, The Pennsylvania State University, Pennsylvania 16802, USA
Search for more papers by this authorErick M. Carreira Prof. Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, HCI H335, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorAndreas Zumbuehl
Laboratorium für Organische Chemie, ETH Hönggerberg, HCI H335, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorDamien Jeannerat Dr.
Départment de Chimie Organique, Université de Genève, 1211 Genève 4, Switzerland
Search for more papers by this authorScott E. Martin Dr.
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorMarc Sohrmann Dr.
Institut für Biochemie, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorPasquale Stano
Department of Chemistry, The Pennsylvania State University, Pennsylvania 16802, USA
Search for more papers by this authorTamas Vigassy Dr.
Laboratorium für Organische Chemie, ETH-Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorDaniel D. Clark
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorStephen L. Hussey Dr.
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorMathias Peter Prof. Dr.
Institut für Biochemie, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorBlake R. Peterson Prof. Dr.
Departement Materialwissenschaft, ETH Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorErnö Pretsch Prof. Dr.
Laboratorium für Organische Chemie, ETH-Hönggerberg, 8093 Zürich, Switzerland
Search for more papers by this authorPeter Walde Prof. Dr.
Department of Chemistry, The Pennsylvania State University, Pennsylvania 16802, USA
Search for more papers by this authorErick M. Carreira Prof. Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, HCI H335, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorWe would like to thank Prof. Dr. Josef Brunner for helpful discussions. E.M.C. is grateful for generous support from the Eidgenössische Technische Hochschule (ETH), Zürich (TH-Gesuch) and from F. Hoffmann LaRoche. B.R.P. acknowledges support from the National Institutes of Health, USA (Grant no. RO1-CA83831).
Graphical Abstract
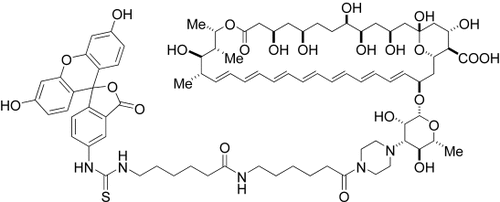
Not only skin deep: A fluorescein–amphotericin B conjugate with a new piperazine linker (see structure) was prepared and used as a probe of the biomembrane and the mechanism of action of amphotericin B both in vivo and in liposomal experiments. The amphotericin B analogue localizes at the membrane of yeast cells but is internalized by mammalian cells.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z460489_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. H. Sternberg, E. T. Wright, M. Oura, Antibiot. Annu. 1955–1956, 566–573;
- 1bB. A. Steinberg, W. P. Jambor, L. O. Suydam, Antibiot. Annu. 1955–1956, 574–578;
- 1cW. Gold, H. A. Stout, J. F. Pagano, R. Donovick, Antibiot. Annu. 1955–1956, 579–586;
- 1dJ. Vandeputte, J. L. Wachtel, E. T. Stiller, Antibiot. Annu. 1955–1956, 587–591.
- 2
- 2aG. Medoff, J. Brajtburg, G. S. Kobayashi, Annu. Rev. Pharmacol. Toxicol. 1983, 23, 303–330;
- 2bJ. Bolard, Biochim. Biophys. Acta 1986, 864, 257–304;
- 2cJ. Brajtburg, W. G. Powderly, G. S. Kobayashi, G. Medoff, Antimicrob. Agents Chemother. 1990, 34, 183–188;
- 2dF. C. Szoka, Jr.,M. Tang, J. Liposome Res. 1993, 3, 363–375;
- 2eS. C. Hartsel, C. Hatch, W. Ayenew, J. Liposome Res. 1993, 3, 377–408;
- 2fJ. Bolard, V. Joly, P. Yeni, J. Liposome Res. 1993, 3, 409–427;
- 2gS. C. Hartsel, J. Bolard, Trends Polym. Sci. 1996, 17, 445–449;
- 2hI. Al-Mohsen, W. T. Hughes, Ann. Saudi Med. 1998, 18, 28–38;
- 2iB. E. Cohen, Int. J. Pharm. 1998, 162, 95–106;
- 2jI. M. Hann, H. G. Prentice, Int. J. Antimicrob. Agents 2001, 17, 161–169;
- 2kS. B. Zotchev, Curr. Med. Chem. 2003, 10, 211–223.
- 3
- 3aW. Huang, Z. Zhang, X. Han, J. Tang, J. Wang, S. Wang, S. Dong, E. Wang, Biophys. J. 2002, 83, 3245–3255;
- 3bB. V. Cotero, S. Rebolledo-Antunez, I. Ortega-Blake, Biochim. Biophys. Acta 1998, 1375, 43–51;
- 3cG. Fujii, J.-E. Chang, T. Coley, B. Steere, Biochemistry 1997, 36, 4959–4968;
- 3dM. Baginski, E. Borowski, J. Mol. Struct. (Theochem) 1997, 389, 139.
- 4
- 4aC. C. Hsu-Chen, D. S. Feingold, Nature 1974, 251, 656–659;
- 4bM. L. Sokol-Anderson, J. Brajtburg, G. Medoff, J. Infect. Dis. 1986, 154, 76–83;
- 4cW. H. Beggs, Antimicrob. Agents Chemother. 1994, 38, 363–364.
- 5
- 5aS. D. Rychnovsky, Chem. Rev. 1995, 95, 2021–2040;
- 5bB. N. Rogers, M. E. Selsted, S. D. Rychnovsky, Bioorg. Med. Chem. Lett. 1997, 7(24), 3177–3182;
- 5cN. Matsumori, N. Yamaji, S. Matsuoka, T. Oishi, M. Murata, J. Am. Chem. Soc. 2002, 124, 4180–4181;
- 5dN. Yamaji, N. Matsumori, S. Matsuoka, R. Oishi, M. Murata, Org. Lett. 2002, 4, 2087–2089;
- 5eS. Matsuoka, N. Matsumori, M. Murata, Org. Biomol. Chem. 2003, 1, 3882–3884;
- 5fN. Matsumori, R. Masuda, M. Murata, Chem. Biodiversity 2004, 1, 346–352.
- 6
- 6aP. Caffrey, S. Lynch, E. Flood, S. Finnan, M. Oliynyk, Chem. Biol. 2001, 8, 713–723;
- 6bB. Byrne, M. Carmody, E. Gibson, B. Rawlings, P. Caffrey, Chem. Biol. 2003, 10, 1215–1224;
- 6cJ. F. Aparicio, P. Caffrey, J. A. Gil, S. B. Zotchev, Appl. Microbiol. Biotechnol. 2003, 61, 179–188.
- 7
- 7aJ. Krüger, E. M. Carreira, Tetrahedron Lett. 1998, 39, 7013–7076;
- 7bJ. Tholander, E. M. Carreira, Helv. Chim. Acta 2001, 84, 613–622.
- 8M. Chéron, B. Cybulska, J. Mazerski, J. Grzybowska, A. Czerwinski, E. Borowski, Biochem. Pharmacol. 1988, 37, 827–836.
- 9J.-P. Salvi, N. Walchshofer, J. Paris, Tetrahedron Lett. 1994, 35, 1181–1184.
- 10J. S. Clark, P. A. Hodgson, M. D. Goldsmith, L. J. Street, J. Chem. Soc. Perkin Trans. 1 2001, 24, 3312–3324.
- 11J. S. Warmus, G. J. Dilley, A. I. Meyers, J. Org. Chem. 1993, 58, 270–271.
- 12M. Daumas, Y. Vo-Quang, L. Vo-Quang, F. Le Goffic, Synthesis 1989, 64–65.
- 13The importance of structural assignment for any subsequent mechanistic investigation led us to confirm the success of the conjugation reaction by using a spectral aliasing technique to acquire high-resolution HSQC and HMBC spectra (see refs. [15, 16]). All but a few signals in the double-bond regions of such 1H, 13C, DEPT, COSY, HSQC, and HMBC spectra were unambiguously assigned to 8.
- 14For the synthesis of an NBD (7-Nitro-2-oxa-1,3-diazol-4-yl)–amphotericin conjugate with an amide linker, see: N. O. Petersen Spectros. Int. J. 1983, 2, 408–414. No characterization data were given for this conjugate; to the best of our knowledge it has not been used for biological studies.
- 15aD. Jeannerat, Magn. Reson. Chem. 2002, 41, 3;
- 15bD. Jeannerat, D. Ronan, Y. Baudry, A. Pinto, J.-P Saulnier, S. Matile, Helv. Chim. Acta, in press ;
- 15cD. Jeannerat, D. Ronan, Y. Baudry, A. Pinto, J.-P Saulnier, S. Matile, Helv. Chim. Acta, in press;
- 15dD. Ronan, Y. Baudry, D. Jeannerat, S. Matile, Org. Lett. 2004, 6, 885–887;
- 15ehttp://rmn.unige.ch/simplealias. We applied the most practical implementation (see ref. [15 c]), which allows the acquisition conditions of heteronuclear experiments (HSQC and HMBC) to be optimized by copying and pasting a list of carbon chemical shifts into an internet base program (see ref. [15 e]). See the Supporting Information for further details.
- 16
- 16aA. Aszalos, A. Bax, N. Burlinson, P. Roller, C. McNeal, J. Antibiot. 1985, 38, 1699–1713;
- 16bP. Sowinski, J. K. Pawlak, E. Borowski, P. Gariboldi, Magn. Reson. Chem. 1992, 30, 275–279;
- 16cP. Sowinski, J. K. Pawlak, E. Borowski, T. Iwashita, J. Antibiot. 1985, 38, 175–180;
- 16dJ. M. Brown, P. J. Sidebottom, Tetrahedron 1981, 37, 1421–1428.
- 17
- 17aP. van Hoogevest, B. de Kruijff, Biochim. Biophys. Acta 1978, 511, 397–407;
- 17bD. S. Orlov, T. Nguyen, R. I. Lehrer, J. Microbiol. Methods 2002, 49, 325–328.
- 18The use of external Ca2+ instead of Na+ ions (as reported previously) seemed to block the pores and yielded no results.
- 19J. Barwicz, P. Tancrède, Chem. Phys. Lipids 1997, 85, 145–155.
- 20V. Dorovska-Taran, R. Wick, P. Walde, Anal. Biochem. 1996, 240, 37–47.
- 21L. A. R. Pioda, V. Stankova, W. Simon, Anal. Lett. 1969, 2, 665–674. For improved ruggedness, ion-selective membranes were used that had a higher than usual (see ref. [22]) content of PVC and a lipophilic salt was added to compensate for the increased resistance.
- 22E. Bakker, P. Bühlmann, E. Pretsch, Chem. Rev. 1997, 97, 3083–3132.
- 23This result may imply a beneficial increase in the therapeutic effect of amphotericin B.
- 24S. L. Hussey, E. He, B. R. Peterson, J. Am. Chem. Soc. 2001, 123, 12 712–12 713.
- 25For flow cytometry data, see the Supporting Information.
- 26PrA is a 57-kDa protein that binds to the invariant Fc fragment of rabbit-derived IgG proteins with a dissociation constant of approximately 60 nM. See ref [24].
- 27L. Cedergren, R. Andersson, B. Jansson, M. Uhlen, B. Nilsson, Protein Eng. 1993, 6, 441–448.
- 28G. Fujii, J.-E. Chang, T. Coley, B. Steere, Biochemistry 1997, 36, 4959–4968.
- 29K. Takeo, J. Gen. Microb. 1985, 131, 309–316.
- 30W. Drabikowski, E. Lagwinska, M. G. Sarzala, Biochim. Biophys. Acta 1973, 291, 61–70.
- 31M. Bagnat, K. Simons, Proc. Natl. Acad. Sci. USA 2002, 99, 14 183–14 188.
- 32V. Wachtler, S. Rajagopalan, M. K. Balasubramanian, J. Cell Sci. 2003, 116, 867–874.
- 33I. Gruda, P. Nadeau, J. Brajtburg, G. Medoff, Biochim. Biophys. Acta 1980, 602, 260–268.
- 34J. Valdez-Taubas, H. R. B. Pelham, Curr. Biol. 2003, 13, 1636–1640.