Carbon Dioxide Fixation by the Cooperative Effect of Organotin and Organotellurium Oxides
Jens Beckmann Dr.
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Current address: Institut für Anorganische und Analytische Chemie, Freie Universität Berlin, Fabeckstrasse 34–36, 14195 Berlin, Germany, Fax: (+49) 30-838-53310
Search for more papers by this authorDainis Dakternieks Prof. Dr.
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorAndrew Duthie
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorNaomi A. Lewcenko
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorCassandra Mitchell
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorJens Beckmann Dr.
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Current address: Institut für Anorganische und Analytische Chemie, Freie Universität Berlin, Fabeckstrasse 34–36, 14195 Berlin, Germany, Fax: (+49) 30-838-53310
Search for more papers by this authorDainis Dakternieks Prof. Dr.
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorAndrew Duthie
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorNaomi A. Lewcenko
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorCassandra Mitchell
Centre for Chiral and Molecular Technologies, Deakin University, School of Biological and Chemcial Sciences, Geelong 3217, Australia
Search for more papers by this authorGraphical Abstract
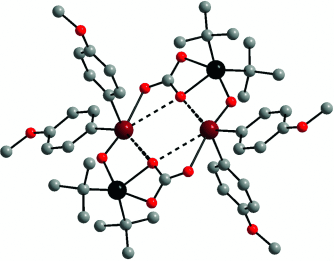
Hypervalency and secondary bonding are the driving forces behind the rapid absorption of gaseous carbon dioxide by two organotellurium and organotin oxides and the unexpected formation of a unique tellurastannoxane cluster (see structure; dark red Te, black Sn, gray C, light red O). The absorption is reversible with the liberation of carbon dioxide being observed at temperatures between 90 and 145 °C.
References
- 1For reviews, see:
- 1aP. G. Jessop, T. Ikariya, R. Noyori, Chem. Rev. 1995, 95, 259;
- 1bD. H. Gibson, Chem. Rev. 1996, 96, 2063;
- 1cA.-A. G. Shaikh, S. Sivaram, Chem. Rev. 1996, 96, 951;
- 1dX. Yin, J. R. Moss, Coord. Chem. Rev. 1999, 181, 27;
- 1eM. Shi, Y.-M. Shen, Curr. Org. Chem. 2003, 7, 737;
- 1fA. Belli Dell'Amico, F. Calderazzo, L. Labella, F. Marchetti, G. Pampaloni, Chem. Rev. 2003, 103, 3857.
- 2
- 2aH. Sato, Bull. Chem. Soc. Jpn. 1967, 40, 410;
- 2bA. J. Bloodworth, A. G. Davies, S. C. Vasishtha, J. Chem. Soc. C 1967, 1309;
- 2cS. J. Blunden, R. Hill, J. N. R. Ruddick, J. Organomet. Chem. 1984, 267, C 5;
- 2dJ. Kümmerlen, A. Sebald, H. Reuter, J. Organomet. Chem. 1992, 427, 309.
- 3
- 3aA. G. Davies, P. G. Harrison, J. Chem. Soc. C 1967, 1313;
- 3bT. Sakakura, Y. Saito, M. Okano, J.-C. Choi, T. Sako, J. Org. Chem. 1998, 63, 7095;
- 3cJ.-C. Choi, T. Sakakura, T. Sako, J. Am. Chem. Soc. 1999, 121, 3793;
- 3dT. Sakakura, J.-C. Choi, Y. Saito, T. Masuda, T. Sako, T. Oriyama, J. Org. Chem. 1999, 64, 4506;
- 3eD. Ballivet-Tkatchenko, O. Douteau, S. Stutzmann, Organometallics 2000, 19, 4563;
- 3fH. Yasuda, J.-C. Choi, S.-C. Lee, T. Sakakura, J. Organomet. Chem. 2002, 659, 133;
- 3gD. Ballivet-Tkatchenko, T. Jerphagnon, R. Ligabue, L. Plasseraud, D. Poinsot, Appl. Catal. A 2003, 255, 93.
- 4H. Puff, W. Schuh, R. Sievers, W. Wald, R. Zimmer, J. Organomet. Chem. 1984, 260, 271.
- 5J. Beckmann, D. Dakternieks, A. Duthie, F. Ribot, M. Schürmann, N. A. Lewcenko, Organometallics 2003, 22, 3257.
- 6For the first work describing molecular tellurastannoxanes, see: J. Beckmann, D. Dakternieks, J. O'Connell, K. Jurkschat, M. Schürmann, Eur. J. Inorg. Chem. 2002, 1484.
- 7
- 7aCrystal data for 1⋅2 CHCl3 (C46H64O12Sn2Te2⋅2 CHCl3): Mr=1540.29, monoclinic, space group P2(1)/c, a=14.4757(8), b=12.2301(7), c=18.3476(10) Å, β=112.5350(10)°, V=3000.2(3) Å3, Z=2, ρcalcd=1.705 mg m−3, MoKα radiation (λ=0.71073 Å), crystal dimensions 0.15×0.20×0.45 mm3. Of 18 492 reflections collected on a Bruker SMART CCD area collector at 130(2) K, 6781 (6286) were observed and used for all calculations (SHELXL 97 implemented in WinGX 2000). After absorption correction the structure was solved by direct methods and refined anisotropically on F2. Final residuals R1=0.0259, wR2=0.0620 (I>2σ(I)); R1=0.0285, wR2=0.0633 (all data). 316 parameters;
- 7bCCDC-233184 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 8For another example of intramolecular secondary Te⋅⋅⋅O interaction, see: D. Dakternieks, R. Di Giacomo, R. W. Gable, B. F. Hoskins J. Am. Chem. Soc. 1988, 110, 6541.
- 9J. Beckmann, K. Jurkschat, B. Mahieu, M. Schürmann, Main Group Met. Chem. 1998, 21, 113.
- 10
- 10aThe tensor analyses were performed according to the method of Herzfeld and Berger: J. Herzfeld, X. Chen in Encyclopedia of Nuclear Magnetic Resonance, Vol. 7 (Eds.: ), Wiley, Chichester, 1996, p. 4362;
- 10bComputer program used: DmFit 2002: D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan, G. Hoatson, Magn. Reson. Chem. 2002, 40, 70;
- 10cDefinitions δiso (ppm)=−σiso=−(σ11+σ22+σ33)/3; ζ (ppm)=σ33−σiso, and η=(σ22−σ11)/(σ33−σiso) where σ11, σ22, and σ33 (ppm) are the principal tensor components of the shielding anisotropy (SA), sorted as follows |σ33−σiso|>|σ11−σiso|>|σ22−σiso|;
- 10dResults obtained: 119Sn ζ 565, η 0.25; σ11 −1637, σ22 −1145, σ33 −755. 125Te ζ −458, η 0.85; σ11 −91, σ22 51, σ33 827 for 1. 119Sn ζ −538, η 0.50; σ11 −118, σ22 151, σ33 824 for 2.
- 10eFor comparison: 119Sn ζ 215, η 0.00 reported for (tBu2SnO)3.[9] 125Te ζ 545, η 0.75 and ζ 570, η 0.60 reported for Ph2TeO.[5] 125Te ζ 210, η 0.00 reported for (p-MeOC6H4)2TeO.[5]
- 11R. L. Lehman, N. G. Glumac, J. S. Gentry, Thermochim. Acta 1998, 316, 1.
- 12T. Fujinami, S. Sato, S. Sakai, Chem. Lett. 1981, 749.