A Mild and Efficient Synthesis of Oxindoles: Progress Towards the Synthesis of Welwitindolinone A Isonitrile†
Joseph M. Ready Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorSarah E. Reisman
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorMakoto Hirata Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorMatthew M. Weiss Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorKazuhiko Tamaki Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorTimo V. Ovaska Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorJohn L. Wood Prof. Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorJoseph M. Ready Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorSarah E. Reisman
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorMakoto Hirata Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorMatthew M. Weiss Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorKazuhiko Tamaki Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorTimo V. Ovaska Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorJohn L. Wood Prof. Dr.
Department of Chemistry, Yale University, Sterling Chemistry Laboratory, PO Box 208107, New Haven, CT 06520–8107, USA, Fax: (+1) 203-432-6144
Search for more papers by this authorWe gratefully acknowledge financial support from Yamanouchi, Merck, Pfizer, and Amgen. J.M.R. is the recipient of an NIH postdoctoral fellowship. K.R. thanks Sankyo Co., LTD.; M.H. thanks Daiso Co., LTD., M.M.W. thanks Bristol Myers Squibb for a graduate fellowship.
Graphical Abstract
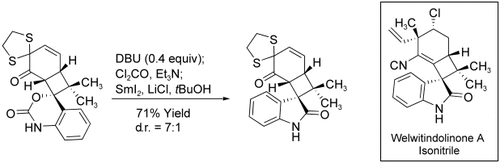
The complete carbon skeleton of welwitindolinone A isonitrile has been prepared by using a [2+2] cycloaddition to establish the bicyclo[4.2.0]octane core and a SmI2-mediated intramolecular reductive cyclization between an enone and an aryl isocyanate to stereoselectively install the spiro-oxindole (see scheme; DBU=1,8-diazabicyclo[5.4.0]undec-7-ene).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53282_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Stratmann, R. E. Moore, R. Bonjouklian, J. B. Deeter, G. M. L. Patterson, S. Shaffer, C. D. Smith, T. A. Smitka, J. Am. Chem. Soc. 1994, 116, 9935–9942;
- 1bJ. I. Jimenez, U. Huber, R. E. Moore, G. M. L. Patterson, J. Nat. Prod. 1999, 62, 569–572.
- 2Synthetic efforts directed toward welwitindolinone alkaloids:
- 2aJ. L. Wood, A. A. Holubec, B. M. Stoltz, M. M. Weiss, J. A. Dixon, B. D. Doan, M. F. Shamji, J. M. Chen, T. P. Heffron, J. Am. Chem. Soc. 1999, 121, 6326–6327;
- 2bH. B. Deng, J. P. Konopelski, Org. Lett. 2001, 3, 3001–3004;
- 2cM. E. Jung, F. Slowinski, Tetrahedron Lett. 2001, 42, 6835–6838;
- 2dP. Lopez-Alvarado, S. Garcia-Granda, C. Alvarez-Rua, C. Avendano, Eur. J. Org. Chem. 2002, 1702–1707.
- 3
- 3aS. Atarashi, J. K. Choi, D. C. Ha, D. J. Hart, D. Kuzmich, C. S. Lee, S. Ramesh, S. C. Wu, J. Am. Chem. Soc. 1997, 119, 6226–6241;
- 3bS. T. Hilton, T. C. T. Ho, G. Pljevaljcic, K. Jones, Org. Lett. 2000, 2, 2639–2641.
- 4A. Madin, L. E. Overman, Tetrahedron Lett. 1992, 33, 4859–4862.
- 5B. ElAli, K. Okuro, G. Vasapollo, H. Alper, J. Am. Chem. Soc. 1996, 118, 4264–4270.
- 6T. Fukuyama, X. Q. Chen, G. Peng, J. Am. Chem. Soc. 1994, 116, 3127–3128.
- 7Y. H. Kim, H. S. Park, D. W. Kwon, Synth. Commun. 1998, 28, 4517–4524.
- 8For the use of
- 8aNiI2: F. Machrouhi, B. Hamann, J. L. Namy, H. B. Kagan, Synlett 1996, 633–634;
- 8bHMPA: J. Inanaga, M. Ishikawa, M. Yamaguchi, Chem. Lett. 1987, 1485–1486;
- 8cLiBr and LiCl: R. S. Miller, J. M. Sealy, M. Shabangi, M. L. Kuhlman, J. R. Fuchs, R. A. Flowers, J. Am. Chem. Soc. 2000, 122, 7718–7722. It has been suggested that addition of LiCl to SmI2 generates SmCl2 in situ, which is a stronger reductant toward ketones.
- 9For complete experimental details, see Supporting Information.
- 10Reactions of α,β-unsaturated ketones with SmI2:
- 10aY. Fujita, S. Fukuzumi, J. Otera, Tetrahedron Lett. 1997, 38, 2121–2124;
- 10bA. Cabrera, R. Le Lagadec, P. Sharma, J. L. Arias, R. A. Toscano, L. Velasco, R. Gavino, C. Alvarez, M. Salmon, J. Chem. Soc. Perkin Trans. 1 1998, 3609–3617.
- 11Y. S. Liu, M. Z. Bei, Z. H. Zhou, K. Takaki, Y. Fujiwara, Chem. Lett. 1992, 1143–1144.
- 12L. Ghosez, M. J. O'Donnell, Pericyclic Reactions, Vol. II, Academic, New York, 1997.
- 13M. L. Gross, D. H. Blank, W. M. Welch, J. Org. Chem. 1993, 58, 2104–2109.
- 14For a discussion of the acidity of cyclobutanones, see: R. J. Cantlin, J. Drake, R. W. Nagorski, Org. Lett. 2002, 4, 2433–2436.
- 15P. Soderman, G. Widmalm, Carbohydr. Res. 1999, 316, 184–186.
- 16A. J. Mancuso, S. L. Huang, D. Swern, J. Org. Chem. 1978, 43, 2480–2482.