Cobalt-Complex-Catalyzed Copolymerization of Ethylene with 2-Aryl-1-methylenecyclopropanes
Daisuke Takeuchi Dr.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan, Fax: (+81) 45-924-5224
Search for more papers by this authorKouhei Anada
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan, Fax: (+81) 45-924-5224
Search for more papers by this authorKohtaro Osakada Prof. Dr.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan, Fax: (+81) 45-924-5224
Search for more papers by this authorDaisuke Takeuchi Dr.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan, Fax: (+81) 45-924-5224
Search for more papers by this authorKouhei Anada
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan, Fax: (+81) 45-924-5224
Search for more papers by this authorKohtaro Osakada Prof. Dr.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan, Fax: (+81) 45-924-5224
Search for more papers by this authorGraphical Abstract
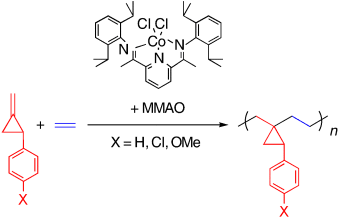
The importance of tolerance: A Cobalt complex with bis(imino)pyridine ligand promotes the alternating copolymerization of ethylene with 2-aryl-1-methylenecyclopropanes to give polymers with regulated C4 repeating units that have a three-membered ring (see scheme). The polymerization is tolerant of functional groups on the three-membered ring such as methoxy and chloro groups.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z52837_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Mecking, L. K. Johnson, L. Wang, M. Brookhart, J. Am. Chem. Soc. 1998, 120, 888–899;
- 1bE. Drent, R. van Dijk, R. van Ginkel, B. van Oort, R. I. Pugh, Chem. Commun. 2002, 744–745.
- 2
- 2aT. R. Younkin, E. F. Connor, J. I. Henderson, S. K. Friedrich, R. H. Grubbs, D. A. Bansleben, Science 2000, 287, 460–462;
- 2bG. M. Benedikt, E. Elce, B. L. Goodall, H. A. Kalamarides, L. H. McIntosh III, L. F. Rhodes, K. T. Selvy, C. Andes, K. Oyler, A. Sen, Macromolecules 2002, 35, 8978–8988.
- 3
- 3aH. Yasuda, M. Furo, H. Yamamoto, A. Nakamura, S. Miyake, N. Kibino, Macromolecules 1992, 25, 5115–5116;
- 3bH. Frauenrath, S. Balk, H. Keul, H. Höcker, Macromol. Rapid Commun. 2001, 22, 1147–1151.
- 4
- 4aT. Miyatake, K. Mizunuma, M. Kakugo, Makromol. Chem. Macromol. Symp. 1993, 66, 203–214;
- 4bT. Uozumi, K. Miyazawa, T. Sano, K. Soga, Macromol. Rapid Commun. 1997, 18, 883–889;
- 4cM. K. Leclerc, R. M. Waymouth, Angew. Chem. 1998, 110, 964–967;
10.1002/(SICI)1521-3757(19980403)110:7<964::AID-ANGE964>3.0.CO;2-T Google ScholarAngew. Chem. Int. Ed. 1998, 37, 922–925.10.1002/(SICI)1521-3773(19980420)37:7<922::AID-ANIE922>3.0.CO;2-G PubMed Web of Science® Google Scholar
- 5
- 5aW. Kaminsky, I. Beulich, M. Arndt-Rosenau, Macromol. Symp. 2001, 173, 211–225;
- 5bJ. Kiesewetter, W. Kaminsky, Chem. Eur. J. 2003, 9, 1750–1758;
- 5cA. L. McKnight, R. M. Waymouth, Macromolecules 1999, 32, 2816–2825.
- 6Polymerization of methylenecyclopropanes by Pd and Ni complexes.
- 6aD. Takeuchi, S. Kim, K. Osakada, Angew. Chem. 2001, 113, 2757–2760;
10.1002/1521-3757(20010716)113:14<2757::AID-ANGE2757>3.0.CO;2-# Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2685–2688;10.1002/1521-3773(20010716)40:14<2685::AID-ANIE2685>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 6bD. Takeuchi, K. Osakada, Chem. Commun. 2002, 646–647;
- 6cD. Takeuchi, K. Anada, K. Osakada, Macromolecules 2002, 35, 9628–9633.
- 7Copolymerization of methylenecyclopropanes with ethylene by early transition metal complexes.
- 7aL. Jia, X. Yang, A. M. Seyam, I. D. L. Albert, P.-F. Fu, S. Yang, T. J. Marks, J. Am. Chem. Soc. 1996, 118, 7900–7913;
- 7bT. R. Jensen, T. J. Marks, Macromolecules 2003, 36, 1775–1778.
- 8
- 8aB. L. Small, M. Brookhart, A. M. A. Bennett, J. Am. Chem. Soc. 1998, 120, 4049–4050;
- 8bG. J. P. Britovsek, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. J. McTavish, G. A. Solan, A. J. P. White, D. J. Williams, Chem. Commun. 1998, 849–850;
- 8cV. C. Gibson, M. J. Humphries, K. P. Tellmann, D. F. Wass, A. J. P. White, D. J. Williams, Chem. Commun. 2001, 2252–2253;
- 8dT. M. Kooistra, Q. Knijnenburg, J. M. M. Smits, A. D. Horton, P. H. M. Budzelaar, A. W. Gal, Angew. Chem. 2001, 113, 4855–4858;
10.1002/1521-3757(20011217)113:24<4855::AID-ANGE4855>3.0.CO;2-9 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4719–4722.10.1002/1521-3773(20011217)40:24<4719::AID-ANIE4719>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 9
- 9aB. L. Small, Organometallics 2003, 22, 3178–3183;
- 9bF. Pelascini, F. Peruch, P. J. Lutz, M. Wesolek, J. Kress, Macromol. Rapid Commun. 2003, 24, 768–771.
- 10Re-addition of 1 to the reaction mixture after consumption of the initially charged monomer (M̄n=8300, M̄w/M̄n=1.16) induces the polymerization to afford a product with an increased molecular weight and a narrow molecular weight distribution (M̄n=26 000, M̄w/M̄n=1.14); this result also indicates that the living polymerization is efficient. A slightly lower efficiency of the initiation than the ideal living polymerization is ascribed to slow or insufficient activation of the Co complex by MMAO. See also: P. Mehrkhodavandi, R. R. Schrock, L. L. Pryor, Organometallics 2003, 22, 4569–4583.
- 11Polymerization of methylenecyclopropane catalyzed by Zr and Ni complexes is proposed to involve the 1,2-insertion of the monomer into the MC σ bond (See refs [6b] and [7]). The 1,2-insertion of monomer is also supported by an end functionalization experiment.
- 12Recent papers on ethylene polymerization by Co catalyst (Ref [8c,d]) suggested that the CoIII complex is most likely the active species in the reaction.
- 13r2=k2,2/k2,ethylene and rethylene=kethylene,ethylene/kethylene,2, in which k2,2 and k2,ethylene denote the rate constants for the insertion reactions of 2 and ethylene into the CoC bond of CoCH2CCH2CH(C6H4OMe-4), respectively.