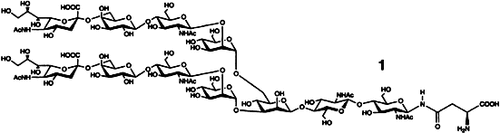
Chemoenzymatic Synthesis of a Sialylated Undecasaccharide–Asparagine Conjugate†‡
Corresponding Author
Dr. Carlo Unverzagt
Institut für Organische Chemie und Biochemie der Technischen Universität München, Lichtenbergstrasse 4, D-85748 Garching (Germany) Fax: Int. code +(89)2891-3210
Institut für Organische Chemie und Biochemie der Technischen Universität München Lichtenbergstrasse 4, D-85748 Garching (Germany) Fax: Int. code +(89)2891-3210Search for more papers by this authorCorresponding Author
Dr. Carlo Unverzagt
Institut für Organische Chemie und Biochemie der Technischen Universität München, Lichtenbergstrasse 4, D-85748 Garching (Germany) Fax: Int. code +(89)2891-3210
Institut für Organische Chemie und Biochemie der Technischen Universität München Lichtenbergstrasse 4, D-85748 Garching (Germany) Fax: Int. code +(89)2891-3210Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft and by the Leonhard-Lorenz-Stiftung, and was presented at the Eighth European Carbohydrate Symposium 1995 in Seville (Spain). I would like to thank the Hoechst AG and Boehringer Mannheim for funding and Prof. Dr. H. Kessler for his generous support.
Dedicated to Professor Hans Paulsen on the occasion of his 75th birthday
Graphical Abstract
A partial structure of many glycoproteins, the complex undecasaccharide–asparagine conjugate 1 was obtained for the first time by total synthesis. Crucial to the synthesis was a combination of modern chemical and enzymatic methods, which reduced the overall number of steps and allowed efficient deprotonation.
References
- 1(a) A. Varki, Glycobiology 1993, 3, 97–130; (b) R. A. Dwek, Chem. Rev. 1996, 96, 683–720.
- 2 T. A. Springer, L. A. Lasky, Nature 1991, 349, 196–197.
- 3(a) M. L. Phillips, E. Nudelman, F. C. A. Gaeta, M. Perez, A. K. Singhal, S.-I. Hakomori, J. C. Paulson, Science, 1990, 250, 1130–1132; (b) G. Walz, A. Aruffo, W. Kolanus, M. Bevilaqua, B. Seed, Science, 1990, 250, 1132–1135; (c) L. A. Lasky, Science 1992, 258, 964–969; (d) A. Giannis, Angew. Chem. 1994, 106, 188–191; Angew. Chem. Intl. Ed. Engl. 1994, 33, 178–180.
- 4 L. D. Powell, D. Sgroi, E. R. Sjoberg, I. Stamencovic, A. Varki, J. Biol. Chem. 1993, 268, 7019–7027.
- 5(a) T. Ogawa, M. Sugimoto, T. Kitajma, K. K. Sadozai, T. Nukada, Tetrahedron Lett. 1986, 27, 5739–5742; (b) Y. Nakahara, S. Shibayama, Y. Nakahara, T. Ogawa, Carbohydr. Res. 1996, 280, 67–84; (c) I. Matsuo, Y. Nakahara, Y. Ito, T. Nukada, Y. Nakahara, T. Ogawa, Bioorg. Med. Chem. 1995, 3, 1455–1463.
- 6 Review: H. Paulsen, Angew. Chem. 1990, 102, 851–857; Angew. Chem. Intl. Ed. Engl. 1990, 29, 823–839.
- 7 H. G. Garg, K. von dem Bruch, H. Kunz in Adv. Carbohydr. Chem. Biochem. 1994, 50, 277–310.
- 8 Review on enzymatic glycosylations: C.-H. Wong, R. L. Halcomb, Y. Ichikawa, T. Kajimoto, Angew. Chem. 1995, 107, 453–474, 569–593; Angew. Chem. Int. Ed. Engl. 1995, 34, 412–432, 521–546.
- 9 C. Unverzagt, Angew. Chem. 1994, 106, 1170–1173; Angew. Chem. Int. Ed. Engl. 1994, 33, 1102–1104.
- 10(a) H. Kunz, W. Günther, Angew. Chem. 1988, 100, 1118–1191; Angew. Chem. Int. Ed. Engl. 1988, 27, 1086–1087; (b) W. Günther, H. Kunz, Carbohydr. Res. 1992, 228, 217–241.
- 11 R. R. Schmidt, W. Kinzy in Adv. Carbohydr. Chem. Biochem, Vol. 50 (Ed.: D. Horton), Academic Press, New York, 1994, p. 21.
- 12 Donor 4[9] was obtained on a 20g scale by the following reaction sequence: (a) 1,3,4,6-tetra-O-acetyl-β-D-mannopyranose and 3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranosyl-trichloroacetimidate, CH2Cl2, molecular sieves 4 Å, BF3-Et2O (59%); (b) hydrazine acetate, DMF (85%); (c) CCl3CN, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), CH2Cl2 (89%).
- 13 H. Paulsen, B. Helpap, Carbohydr. Res. 1991, 216, 289–313.
- 14 F. Cramer, N. Hennrich, Chem. Ber. 1961, 94, 976–989.
- 15 S. Hünig, H. R. Müller, W. Thier, Angew. Chem. 1965, 77, 368–377; Angew. Chem. Int. Ed. Engl. 1965, 4, 271–280.
- 16 O. Kanie, S. C. Crawley, M. M. Palcic, O. Hindsgaul, Carbohydr. Res. 1993, 243, 139–164.
- 17 Preparation of 2: M. Green, J. Bermann, Tetrahedron Lett. 1990, 31, 5851–5852.
- 18 Synthesis of the GlcNAc-β-Asn bond without use of noble metals: (a) A. Y. Khorlin, S. E. Zurabayan, R. G. Macharadze, Carbohydr. Res. 1990, 85, 201–208; (b) T. Inazu, K. Kobayashi Synlett. 1993, 869–870; (c) S. T. Cohen-Anisfield, P. T. Lansbury, Jr., J. Am. Chem. Soc. 1993, 115, 10531–10537.
- 19(a) A. Bayley, D. N. Standring, J. R. Knowles, Tetrahedron Lett. 1978, 19, 3633–3634; (b) According to this procedure an anthracene sulfonamide was cleaved with concomitant reduction of an azido group: J. Y. Roberge, X. Beebe, S. J. Danishefsy, Science 1995, 269, 202–204.
- 20(a) L. J. Berliner, M. E. Davis, K. E. Ebner, T. A. Beyer, J. E. Bell, Mol. Cell Biochem, 1984, 62, 37–42; (b) C. Augé, R. Fernandez-Fernandez, C. Gautheron, Carbohydr. Res. 1990, 200, 257–268; (c) J. Lehmann, Carbohydr. Res. 1994, 252, 325–332.
- 21(a) C. Unverzagt, H. Kunz, J. C. Paulson, J. Am. Chem. Soc. 1990, 112, 9308–9309; (b) C. Unverzagt, S. Kelm, J. C. Paulson, Carbohydr. Res. 1994, 251, 285–301; (c) We were informed that the influence of alkaline phosphatase in galactosylations was also examined by A. Redlitz (Diplomarbeit, Berlin, 1988). (d) Glycosyl transferases and nucleotide sugars were obtained from Sigma, alkaline phosphatase (calf intestine) from Boehringer Mannheim.
- 22 Review: H. Schachter, Biochem. Cell. Biol. 1986, 64, 163–181.
- 23
1: 5.6 mg yield (86% based on 13); [α]
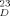 = −9.1° (0.5, H2O); 1H NMR (500 MHz, D2O): δ = 5.19 (d, J1, 2 < 1.0 Hz, 1 H, H-14), 5.12 (d, J1, 2 = 9.7 Hz, 1 H, H-11), 5.01 (d, J1, 2 < 1.0 Hz, 1 H, H-14′), 4.83 (d, J1, 2 < 1.0 Hz, 1 H, H-13), 4.69–4.65 (m, 3 H, H-12, H-15, H-15′), 4.505, 4.502 (2d, J1, 2 = 7.8 Hz, 2 H, H-16, H-16′), 4.31 (dd, J2, 3 = 1.9 Hz, H-23), 4.25 (dd, J2, 3 = 1.9 Hz, H-24), 4.17 (dd, J2, 3 = 1.9 Hz, H-24′), 3.00 (dd, Jgem = 17.2 Hz, Jvic = 4.2 Hz, 1 H, β-CHa-Asn), 2.92 (dd, Jvic = 7.0 Hz, 1 H, β-CHb-Asn), 2.76–2.71 (m, 2H, H-3eqN, H-3eqN′), 2.14, 2.13, 2.09, 2.07, 2.06 (5s, 18H, NAc), 1.78 (t, Jgem = 12.1 Hz, 2 H, H-3axN, H-3axN′). 13C NMR (125 MHz, D2O, [D6] DMSO as internal standard; the chemical shifts were determined by an HMQC spectum): δ = 104.8 C-16, C-16′, 102.6 C-12, 101.8 C-13, 100.7 C-14, 100.6 C-15, C-15′, 98.1 C-14′, 81.9 C-45, C-45′, 81.8 C-33, 80.9 C-42, 80.2 C-41, 79.4 C-11, 77.8 C-24, 77.6 C-24′, 77.5 C-51, 75.7 C-52, C-52, C-53, C-55, C-55′, 75.0 C-56, C-56′, 74.9 C-54, 74.1 C-54′, 74.0 C-31, 73.8 C-36, C-36′, C-3N, C-3N′, 73.3 C-32, C-35, C-35′, 73.0 C-8N, C-8N′, 72.0 C-26, C-26′, 71.4 C-23, 70.7 C-34, C-34′, 69.7 C-7N, C-7N′, 69.6 C-46, C-46′, 69.4 C-4N, C-4N′, 68.6 C-44, C-44′, 67.1 C-63, 67.0 C-43, 64.6 C-66, C-66′, 64.0 C-9N, C-9N′, 62.9 C-64, C-64′, 61.5 C-65, C-65′, 61.2 C-62, 61.1 C-61, 56.2 C-22, 55.9 C-25, C-25′, 54.9 C-21, 53.2 C-5N, C-5N′, 52.1 αC-Asn, 39.0 C-3N, C-3N′, 36.3 βC-Asn, 23.9, 23.8, 23.7 NAc.
= −9.1° (0.5, H2O); 1H NMR (500 MHz, D2O): δ = 5.19 (d, J1, 2 < 1.0 Hz, 1 H, H-14), 5.12 (d, J1, 2 = 9.7 Hz, 1 H, H-11), 5.01 (d, J1, 2 < 1.0 Hz, 1 H, H-14′), 4.83 (d, J1, 2 < 1.0 Hz, 1 H, H-13), 4.69–4.65 (m, 3 H, H-12, H-15, H-15′), 4.505, 4.502 (2d, J1, 2 = 7.8 Hz, 2 H, H-16, H-16′), 4.31 (dd, J2, 3 = 1.9 Hz, H-23), 4.25 (dd, J2, 3 = 1.9 Hz, H-24), 4.17 (dd, J2, 3 = 1.9 Hz, H-24′), 3.00 (dd, Jgem = 17.2 Hz, Jvic = 4.2 Hz, 1 H, β-CHa-Asn), 2.92 (dd, Jvic = 7.0 Hz, 1 H, β-CHb-Asn), 2.76–2.71 (m, 2H, H-3eqN, H-3eqN′), 2.14, 2.13, 2.09, 2.07, 2.06 (5s, 18H, NAc), 1.78 (t, Jgem = 12.1 Hz, 2 H, H-3axN, H-3axN′). 13C NMR (125 MHz, D2O, [D6] DMSO as internal standard; the chemical shifts were determined by an HMQC spectum): δ = 104.8 C-16, C-16′, 102.6 C-12, 101.8 C-13, 100.7 C-14, 100.6 C-15, C-15′, 98.1 C-14′, 81.9 C-45, C-45′, 81.8 C-33, 80.9 C-42, 80.2 C-41, 79.4 C-11, 77.8 C-24, 77.6 C-24′, 77.5 C-51, 75.7 C-52, C-52, C-53, C-55, C-55′, 75.0 C-56, C-56′, 74.9 C-54, 74.1 C-54′, 74.0 C-31, 73.8 C-36, C-36′, C-3N, C-3N′, 73.3 C-32, C-35, C-35′, 73.0 C-8N, C-8N′, 72.0 C-26, C-26′, 71.4 C-23, 70.7 C-34, C-34′, 69.7 C-7N, C-7N′, 69.6 C-46, C-46′, 69.4 C-4N, C-4N′, 68.6 C-44, C-44′, 67.1 C-63, 67.0 C-43, 64.6 C-66, C-66′, 64.0 C-9N, C-9N′, 62.9 C-64, C-64′, 61.5 C-65, C-65′, 61.2 C-62, 61.1 C-61, 56.2 C-22, 55.9 C-25, C-25′, 54.9 C-21, 53.2 C-5N, C-5N′, 52.1 αC-Asn, 39.0 C-3N, C-3N′, 36.3 βC-Asn, 23.9, 23.8, 23.7 NAc.
- 24 L. Dorland, J. Haverkamp, B. L. Schut, J. F. G. Vliegenthart, FEBS Lett. 1977, 77, 15–20.
- 25 ESI-MS: (50% 0.01 M NH4OAc, 50% acetonitrile): C88H144N8O64 Mr (calcd) = 2336.8; Mr (found) = 2337.8 (M + 1).
- 26 R. R. Townsend, E. Hilliker, Y.-T. Li, R. A. Laine, W. R. Bell, Y. C. Lee, J. Biol. Chem. 1982, 257, 9704–9710.