Lanthanoid Complexes with [(dad)Li] Ligands—New Starting Materials for Organolanthanoid Chemistry†
Dr. Helmar Görls
Max-Planck-Gruppe für “Co 2 -Chemie” am Institut für Anorganische Chemie der Universität Jena (FRG)
Search for more papers by this authorDr. Bernhard Neumüller
Fachbereich Chemie der Universität Marburg (FRG)
Search for more papers by this authorDr. Annett Scholz
Institut für Anorganische Chemie der Universität Halle-Wittenberg Geusaer Strasse, D-06217 Merseburg (FRG) Telefax: Int. code + (3461)46-2370
Search for more papers by this authorCorresponding Author
Dr. Joachim Scholz
Institut für Anorganische Chemie der Universität Halle-Wittenberg Geusaer Strasse, D-06217 Merseburg (FRG) Telefax: Int. code + (3461)46-2370
Institut für Anorganische Chemie der Universität Halle-Wittenberg Geusaer Strasse, D-06217 Merseburg (FRG) Telefax: Int. code + (3461)46-2370Search for more papers by this authorDr. Helmar Görls
Max-Planck-Gruppe für “Co 2 -Chemie” am Institut für Anorganische Chemie der Universität Jena (FRG)
Search for more papers by this authorDr. Bernhard Neumüller
Fachbereich Chemie der Universität Marburg (FRG)
Search for more papers by this authorDr. Annett Scholz
Institut für Anorganische Chemie der Universität Halle-Wittenberg Geusaer Strasse, D-06217 Merseburg (FRG) Telefax: Int. code + (3461)46-2370
Search for more papers by this authorCorresponding Author
Dr. Joachim Scholz
Institut für Anorganische Chemie der Universität Halle-Wittenberg Geusaer Strasse, D-06217 Merseburg (FRG) Telefax: Int. code + (3461)46-2370
Institut für Anorganische Chemie der Universität Halle-Wittenberg Geusaer Strasse, D-06217 Merseburg (FRG) Telefax: Int. code + (3461)46-2370Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft.
Graphical Abstract
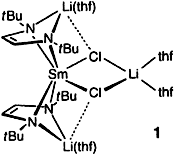
Easy to synthesize and highly soluble are the new (1,4-diaza-1,3-diene) complexes 1 and 2, which can be considered as alternative starting materials to the frequently used cyclopentadienyl lanthanoid halides in organolanthanoid chemistry. The similarity of Cp ligands and the [(dad)Li]- unit is apparent in the structure of 1 on the right; complexes 2 have a different structure as a result of the smaller ionic radius of the lanthanoid.
References
- 1 Reviews on organolanthanoid chemistry: (a) H. Schumann, Angew. Chem. 1984, 96, 475–493; Angew. Chem. Int. Ed. Engl. 1984, 23, 474–493; (b) W. J. Evans, Adv. Organomet. Chem. 1985, 24, 131–177; (c) Polyhedron 1987, 6, 803–835.
- 2(a) Tritox [{Me3C}3CO]−: W. A. Herrmann, R. Anwander, W. Scherer, Chem. Ber. 1993, 126, 1533–1539; (b) M. Wedler, J. W. Gilje, U. Pieper, D. Stalke, M. Noltemeyer, F. T. Edelmann, Chem. Ber. 1991, 124, 1163–1165; (c) silox [(Me3C)3SiO]−: W. A. Herrmann, R. Anwander, M. Kleine, W. Scherer, Chem. Ber. 1992, 125, 1971–1979; (d) scopionate [HB(pz)3]−: M. V. Steiner, J. Takats, J. Am. Chem. Soc. 1983, 105, 410–415; (e) L. Hasinoff, J. Takats, X. W. Zhang, A. H. Bond, R. D. Rodgers, J. Am. Chem. Soc. 1995, 116, 8833–8834; (f) benzamidinate [PhC(NSiMe3)2]−: M. Wedler, M. Noltemeyer, U. Pieper, H.-G. Schmidt, D. Stalke, F. T. Edelmann, Angew. Chem. 1990, 102, 941–943; Angew. Chem. Int. Ed. Engl. 1990, 29, 894–896; (g) A. Recknagel, F. Knösel, H. Gornitzka, M. Noltemeyer, F. T. Edelmann, J. Organomet. Chem. 1991, 417, 363–375; (h) M. Wedler, A. Recknagel, J. W. Gilje, M. Noltemeyer, F. T. Edelmann, J. Organomet. Chem. 1992, 426, 295–306; (i) M. Wedler, F. Knösel, U. Pieper, D. Stalke, F. T. Edelmann, H.-D. Amberger, Chem. Ber. 1992, 125, 2171–2181.
- 3(a)
(dad)E: EC:
A. J. Arduengo III,
R. J. Harlow,
M. Kline,
J. Am. Chem. Soc.
1991,
113, 361–363;
(b)
E = Si:
M. Denk,
R. K. Hayashi,
R. West,
J. Chem. Soc. Chem. Commun. 1995, 33–34;
(c)
E = Ge:
W. A. Herrmann,
M. Denk,
J. Behm,
W. Scherer,
F.-J. Klingan,
H. Bock,
B. Solouki,
M. Wagner,
Angew. Chem.
1992,
104, 1489–1492;
Angew. Chem. Int. Ed. Engl.
1992,
31, 1485–1488; (dad)2M:
(d)
M = Li, Mg, Zn:
M. G. Gardiner,
G. R. Hanson,
M. J. Henderson,
F. C. Lee,
C. L. Raston,
Inorg. Chem.
1995,
33, 2456–2461;
10.1021/ic00089a024 Google Scholar(e) M = Al: F. G. N. Cloke, C. I. Dalby, M. J. Henderson, P. B. Hitchcock, C. H. L. Kennard, R. N. Lamb, C. L. Raston, J. Chem. Soc. Chem. Commun. 1990, 1394–1396; (f) M = Ga: F. G. N. Cloke, G. R. Hanson, M. J. Henderson, P. B. Hitchcock, C. L. Raston, J. Chem. Soc. Chem. Commun. 1989, 1002–1003.
- 4(a) (dad)2Ti: H. tom Dieck, J. Rieger, G. Fendesak, Inorg. Chim. Acta 1990, 177, 191–197; (b) (dad)2M (M = Zr, Hf): C. Trompke, Dissertation, Universität Hamburg, 1992; (c) (dad)M(BH4M2 (M = Ti, Zr): W. A. Herrmann, M. Denk, W. Scherer, F.-R. Klingan, J. Organomet. Chem. 1993, 444, C21–C24.
- 5(a)
(dad)3Ln (Ln = Y, Nd, Sm, Yb):
F. G. N. Cloke,
H. C. de Lemos,
A. A. Sameh,
J. Chem. Soc. Chem. Commun. 1986, 1344–1345;
(b)
(dad)3Sm:
F. G. N. Cloke,
Chem. Soc. Rev. 1993, 17–24;
(c)
(dad)2Yb:
A. A. Trifonov,
A. V. Protchenko,
V. K. Cnerkasov,
M. N. Bochkarev,
Metallorgan. Khim.
1989,
2, 471;
(d)
[Cp
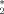 Sm(dad)]:
A. Recknagel,
M. Noltemeyer,
F. T. Edelmann,
J. Organomet. Chem.
1991,
410, 53–61;
(e)
After the completion of our work the Yb complex [Cp2Yb(μ-dad)Li(dme)] was published; the central Yb(μ-dad)Li-structural unit is comparable with that of 3a:
A. A. Trifonov,
L. N. Zakharov,
M. M. Bochkarev,
Yu. T. Struchkov,
Izv. Akad. Nauk SSSR Ser. Khim. 1995, 148–151.
Sm(dad)]:
A. Recknagel,
M. Noltemeyer,
F. T. Edelmann,
J. Organomet. Chem.
1991,
410, 53–61;
(e)
After the completion of our work the Yb complex [Cp2Yb(μ-dad)Li(dme)] was published; the central Yb(μ-dad)Li-structural unit is comparable with that of 3a:
A. A. Trifonov,
L. N. Zakharov,
M. M. Bochkarev,
Yu. T. Struchkov,
Izv. Akad. Nauk SSSR Ser. Khim. 1995, 148–151.
- 6(a) 1: J. M. Kliegman, R. K. Barnes, Tetrahedron 1970, 26, 2555–2560; 1H NMR (300 MHz, [D8]THF, 25 °C): δ = 7.83 (s. 2H; NCH), 1.20 (s, 18H, CMe3); 13C NMR (75 MHz, [D8]THF, 20 °C): δ = 158.14 (dd, 1 J(C,H) = 161.1 Hz, 2 J(C,H) = 10.6 Hz; NCh), 58.40 (s; CMe3), 29.72 (q, 1 J(C,H) = 125.8 Hz; CMe3); (b) 2: 1 (5.20 g, 30.90 mmol) was dissolved in THF (100 mL) and treated with lithium sand (0.429 g, 61.80 mmol) at room temperature. The reaction mixture changed to orange red and was stirred for 24 h. Subsequently the solvent was removed under vacuum and the residue taken up in a mixture of diethyl ether/n-pentane (50 mL). Compound 2 crystallized at −20 °C as a THF adduct of the composition [{Li(thf)2}2{( tBu)NCHCHN( tBu)}]. 1H NMR (300 MHz, [D8]THF, −80 °C): δ = 5.16 (s. 2H; NCH), 1.00 (s, 18H; CMe3); (75 MHz, [D8]THF, −80 °C): δ = 113.79 (dd, 1 J(C,H) = 154.8 Hz, 2 J(C,H) = 11.4; Hz; NCN), 51.13 (s; CMe3), 33.35 (q, 1 J(C,H) = 124.4 Hz; CMe3).
- 7X-ray structure analysis of 3b: C28H58ClN4O2Li2Lu, M = 707.08 g mol−1, crystal dimensions 0.40 × 0.40 × 0.38 mm3, triclinic, space group P1, a = 1063.1(2), b = 1202.8(2), c = 1422.4(3) pm, α = 81.40(3), β = 83.06(3), γ = 81.07(3)°, V = 1767.7(6) × 106 pm3, Z = 2, ρcalcd = 1.328 g cm−3, Enraf-Nonius CAD-4 diffractometer, MoKα radiation, λ = 71.073 pm, graphite monochromator, T = 183(2) K, μ = 2.895 mm−1, scan range 2.59 < ω < 25.97°, 7260 measured reflections, 6915 symmetry independent reflections, 6906 of which were observed (I > 2σ(I)), 343 refined parameters, R1 = 0.0430, wR2 = 0.0874. X-ray structure analysis of 3d: C36H72Cl2N4O4Li3Sm, M = 867.08 g mol−1, crystal dimensions 0.50 × 0.45 × 0.45 mm3, monoclinic, space group C2/c, a = 1649.4(2), b = 1270.9(2), c = 2238.4(4) pm, β = 108.67(1)°, V = 4445(1) × 106 pm3, Z = 4, ρcalcd = 1.296 g cm−3, Enraf-Nonius CAD-4 diffractometer, MoKα radiation, λ = 71.071 pm, graphite monochromator, T = 193 K, scan range 6.2 < 2θ < 52.7°, 4503 symmetry independent reflections, 3406 of which were observed (|Fo| > 4σ | Fo|), R1 = 0.0485, wR2 = 0.1127. The two split positions C611 (occupation factor 0.8, anisotropicic) and C612 (occupation factor 0.2, isotropic) were calculated for the disordered C atom C61. All H atoms except H2 and H3 were calculated for an ideal geometry and were refined with mutual deflection parameters. H2 and H3 were refined free. Structure representations with SCHAKAL 92 (E. Keller, Universität Freiburg, 1992). Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (FRG) on quoting the depository number CSD-58697.
- 8 J. Scholz, B. Richter, R. Goddard, C. Krüger, Chem. Ber. 1993, 126, 57–61.
- 9(a)
A distance of 2.50 Å has been discussed as an upper limit for a Li-C interaction:
W. Zarges,
M. Marsch,
K. Harms,
W. Koch,
G. Frenking,
G. Boche,
Chem. Ber.
1991, 124
543–549;
(b)
The Ln–(CC) distances in 3b and 3d are comparable with the average Ln–CCp* distances of Cp
 LnIII complexes (Sm: 2.68(1)–2.80(1) Å; Lu 2.63(2)–2.66(2) Å [10]).
LnIII complexes (Sm: 2.68(1)–2.80(1) Å; Lu 2.63(2)–2.66(2) Å [10]).
- 10
W. J. Evans,
S. E. Foster,
J. Organomet. Chem.
1992,
433, 79–94.
Whereas in the [Cp
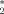 Ln(μ-X)2ML2] complexes the X-Ln-X′ planes are almost perpendicular to the Cp*-Ln-Cp* planes, in 3d the C11-Sm-C11a plane is only tilted towards the center( 1)-Ln-center( 1)′ plane by 55° because of the short Li1–Cl1 and Li1a–Cl1a contacts, respectively.
Ln(μ-X)2ML2] complexes the X-Ln-X′ planes are almost perpendicular to the Cp*-Ln-Cp* planes, in 3d the C11-Sm-C11a plane is only tilted towards the center( 1)-Ln-center( 1)′ plane by 55° because of the short Li1–Cl1 and Li1a–Cl1a contacts, respectively.
- 11 F. A. Cotton, G. Wilkinson, Advanced Inorganic Chemistry, 4. edition, Wiley, New York, 1980.
- 12(a) Likewise for steric reasons the Li is only three-coordinate in: [{Me3Si)2NLi}2 · 2 Et2O]: M. F. Lappert, M. J. Slade, A. Singh, J. L. Atwood, R. D. Rogers, R. Shakir, J. Am. Chem. Soc. 1983, 105, 302–304; (b) [{(Me3Si)2NLi}2 · 2 THF]: L. M. Engelhardt, B. S. Jolly, P. C. Punk, C. L. Raston, B. W. Skelton, A. H. White, Aust. J. Chem. 1986, 39, 133; (c) P. G. Willard, M. A. Nichols, J. Am. Chem. Soc. 1991, 113, 9671–9673.
- 13(a) J. Scholz, M. Dlikan, D. Ströhl, A. Dietrich, H. Schumann, K.-H. Thiele, Chem. Ber. 1990, 123, 2279–2285; (b) R. Goddard, C. Krüger, G. A. Hadi, K.-H. Thiele, J. Scholz, Z. Naturforsch. B 1995, 49, 519–528; (c) see also [4b, c].
- 14 4: orange-yellow crystals; 1H NMR (300 MHz, [D8]THF, 25 °C): δ = 5.94 (s, 5H; Cp), 5.52 (s, 2H; NCH), 5.50 (s, 5H; Cp), 1.26 (s, 18H; CMe3); 13C NMR (75 MHz, [D8]THF, 20 °C): δ = 108.74 (d, 1 J(C,H) = 170.6 Hz; Cp), 107.86 (dd, 1 J(C,H) = 161.7 Hz, 2 J(C,H) = 9.7 Hz; NCH), 101.64 (d, 1 J(C,H) = 169.9 Hz; Cp), 58.00 (s; CMe3), 32.12 (q. 1 J(C,H) = 125.0 Hz; CMe3). -5: pale yellow crystals; 1H NMR (300 MHz, [D8]THF, 20 °C): δ = 6.36 (s, 5H; Cp), 5.87 (s, 2H; NCH), 1.24 (s, 18H; CMe3); 13C NMR (75 MHz, [D8]THF, 25 °C): δ = 111.54 (d, 1 J(C,H) = 172.0 Hz; Cp), 105.31 (dd, 1 J(C,H) = 168.7 Hz, 2 J(C,H) = 10.2 Hz; NCH), 59.46 (s; CMe3), 31.56 (q, 1 J(C,H) = 125.4 Hz; CMe3).