μ-Nitridodiiron Complexes with Asymmetric [FeIVN-FeIII]4+ and Symmetric [FeIVNFeIV]5+ Structural Elements†
Dipl.-Chem. Thomas Jüstel
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Search for more papers by this authorDipl.-Chem. Thomas Weyhermüller
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Search for more papers by this authorCorresponding Author
Prof. Dr. Karl Wieghardt
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Max-Planck-Institut für Strahlenchemie Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951Search for more papers by this authorDipl.-Phys. Marek Lengen
Institut für Physik, Universität Lübeck (FRG)
Search for more papers by this authorProf. Dr. Alfred X. Trautwein
Institut für Physik, Universität Lübeck (FRG)
Search for more papers by this authorDr. Peter Hildebrandt
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Search for more papers by this authorDipl.-Chem. Thomas Jüstel
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Search for more papers by this authorDipl.-Chem. Thomas Weyhermüller
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Search for more papers by this authorCorresponding Author
Prof. Dr. Karl Wieghardt
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Max-Planck-Institut für Strahlenchemie Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951Search for more papers by this authorDipl.-Phys. Marek Lengen
Institut für Physik, Universität Lübeck (FRG)
Search for more papers by this authorProf. Dr. Alfred X. Trautwein
Institut für Physik, Universität Lübeck (FRG)
Search for more papers by this authorDr. Peter Hildebrandt
Max-Planck-Institut für Strahlenchemie, Stiftstrasse 34-36, D-45470 Mülheim an der Ruhr (FRG) Telefax: Int. code + (208) 3063951
Search for more papers by this authorThis work was supported by the Fonds der Chemischen Industrie and the Deutsche Forschungsgemeinschaft.
Graphical Abstract
Very strong antiferromagnetic coupling exists between the Fe centers in the N-bridged complexes 1 and 2. In 1 (shown on the right), which contains a symmetrical [FeIV = N = FeIV] unit, this results in an S = 0 ground state, while in 2, which has an unsymmetrical FeIII–N = FeIV bridge, it leads to an S = 3/2 ground state. Compound 2 forms in the photolysis of [FeIII(cat)N3] and can be oxidized with Br2 to give 1. L = trimethyltriazacyclononane, cat = tetrachlorocatecholate(2−).

References
- 1 K. Dehnicke, J. Strähle, Angew. Chem. 1992, 104, 978; Angew. Chem. Int. Ed. Engl. 1992, 31, 955.
- 2 D. A. Summerville, I. A. Cohen, J. Am. Chem. Soc. 1976, 98, 1747.
- 3 J. W. Buchler, C. Dreher, Z. Naturforsch. B 1984, 39, 222.
- 4 W. R. Scheidt, D. A. Summerville, I. A. Cohen, J. Am. Chem. Soc. 1976, 98, 6623.
- 5(a) K. Kadish, L. A. Bottomley, J. G. Brace, N. Winograd, J. Am. Chem. Soc. 1980, 102, 4341; (b) K. Tatsumi, R. Hoffmann, M. H. Whangbo, J. Chem. Soc. Chem. Commun. 1980, 509; (c) K. Tatsumi, R. Hoffmann, J. Am. Chem. Soc. 1981, 103, 3328.
- 6 L. A. Bottomley, B. B. Garrett, Inorg. Chem. 1982, 21, 1260.
- 7For an example, see Table in C. Ercolani, S. Hewage, R. Heucher, G. Rossi, Inorg. Chem. 1993, 32, 2975.
- 8 M. B. Robin, P. Day, Adv. Inorg. Chem. Radiochem. 1967, 10, 247.
- 9(a) L. A. Bottomley, J.-N. Gorce, V. L. Goedken, C. Ercolani, Inorg. Chem. 1985, 24, 3733; (b) K. M. Kadish, R. K. Rhodes, L. A. Bottomley, H. M. Goff, Inorg. Chem. 1981, 20, 3195.
- 10 C. Ercolani, M. Gardini, G. Pennesi, G. Rossi, U. Russo, Inorg. Chem. 1988, 27, 422.
- 11(a) D. R. English, D. N. Hendrickson, K. S. Suslick, Inorg. Chem. 1985, 24, 122; (b) B. Moubaraki, D. Benlian, A. Baldy, M. Pierrot, Acta Crystallogr. Sect. C 1989, 45, 393.
- 12 D. M. Kurtz, Jr., Chem. Rev. 1990, 90, 585, and references cited therein.
- 13(a)
E. Vogel,
S. Will,
A. Schulze-Tilling,
L. Neumann,
J. Lex,
E. Bill,
A. X. Trautwein,
K. Wieghardt,
Angew. Chem.
1995,
106, 771;
10.1002/ange.19941060708 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 33, 731; (b) M. Lausmann, I. Zimmer, J. Lex, H. Lueken, K. Wieghardt, E. Vogel, Angew. Chem. Int. Ed. Engl. 1995, 106, 776;10.1002/ange.19941060709 Google Scholarand Angew. Chem. Int. Ed. Engl. 1995, 33, 736.
- 14 An analogous complex, [Fe(tacn)(cat')CI] (tacn = 1,4,7-triazacyclononane, cat' = 3,5-di-tert-butylcatecholate) has recently been reported: A. Dei, D. Gatteschi, L. Pardi, Inorg. Chem. 1993, 32, 1389.
- 15
3·2 toluene: C44H58CI8Fe2N6O5, crystals grown from acetonitrile/toluene; monoclinic, space group P21/ m, a = 10.563(6), b = 12.617(3), c = 19.659(9) Å, β = 100.31(9)°, Z = 2; ρcalcd = 1.48 gcm−3; μMo = 1.026 mm−1; 3297 observed reflections ( I ≥ 2.0σ( I)); empirical absorption correction, ψ scans; 2λmax = 55°; R = 0.065; wR = 0.066; maximum residual electron density 0.67 e Å−3. 4: C30H42Cl8Fe2N7O4, crystals grown from dichloromethane; monoclinic, space group P21/ n, a = 8.807(1), b = 17.024(2), c = 13.011(2) Å, β = 90.78(3)°, Z = 2; ρcalcd = 1.63 gcm−3; μMo = 1.337 mm−1; 2880 observed reflections ( I ≥ 2.0σ( I)); empirical absorption correction, ψ scans; 2λmax = 55°; R = 0.048; wR = 0.048; maximum residual electron density 0.53 e Å−3. 5°CH3CN: C32H45BrCl8Fe2N8O4, crystals grown from acetonitrile/toluene; monoclinic, space group C2/ c, a = 20.845(7), b = 13.781(6), c = 15.408(5) Å, β = 99.80(2)°, Z = 4; ρcalcd = 1.646 gcm−3; μMo = 2.117 mm−1; 2586 observed reflections ( I ≥ 2.0σ( I)); empirical absorption correction, ψ scans; 2λmax = 55°; R = 0.060; wR = 0.058; maximum residual electron density 0.96 e Å−3, located in the vicinity of the disordered acetonitrile molecule, which was refined using a split-atom model. The data collection was performed at 293 K using a Siemens P4 diffractometer, employing monochromatic MoKα radiation. The structures were solved using Patterson and difference Fourier syntheses and were refined against F2 for all observed, independent reflections. Heavy atoms were given anisotropic temperature factors. H atoms, which were placed in calculated positions, were given isotropic temperature factors; wR = [Σ w( F
 – F
– F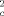 )2/Σ w(F
)2/Σ w(F )2]1/2. The Siemens-SHELXTL-PLUS program package (PC version) by G. M. Sheldrick (Universität Göttingen) was used for the structure solutions and refinements. Further details on the crystal structure investigations may be obtained from the fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen, on quoting the depository number CSD-58767.
)2]1/2. The Siemens-SHELXTL-PLUS program package (PC version) by G. M. Sheldrick (Universität Göttingen) was used for the structure solutions and refinements. Further details on the crystal structure investigations may be obtained from the fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen, on quoting the depository number CSD-58767.
- 16 The Fe-N distance of 1.50(1) Å is unrealistically short; a distance of about 1.60 Å is more likely to be correct.
- 17 O. Carugo, C. B. Castellani, K. Djinovic, M. Rizzi, J. Chem. Soc. Dalton Trans. 1992, 837.
- 18 J. A. Hartmann, R. L. Rardin, P. Chaudhuri, K. Pohl, K. Wieghardt, B. Nuber, J. Weiss, G. C. Papaefthymiou, R. B. Frankel, S. J. Lippard, J. Am. Chem. Soc. 1987, 109, 7387.
- 19 Whether the spin density of two unpaired electrons is localized on the FeIV ion or delocalized over the NFe moiety can only be determined by magnetic Mössbauer measurements and MO calculations.





