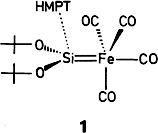
Synthesis and Structure of [(OC)4Fe Si(OtBu)2·HMPT], a Donor-Stabilized Silanediyl (“Silylene”) Complex†
Corresponding Author
Dr. Christian Zybill
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)Search for more papers by this authorDr. Gerhard Müller
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorCorresponding Author
Dr. Christian Zybill
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)Search for more papers by this authorDr. Gerhard Müller
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorHMPT = hexamethyl phosphoric triamide.
Graphical Abstract
The first stable silanediyl complex 1 is monomeric both in solution and in the crystalline state. 1 has a polar SiFe bond and is stabilized by adduct formation with hexamethylphosphoric triamide (HMPT). As expected, the silanediyl ligand occupies an apical position of the trigonal-bipyramidally coordinated iron atom.
References
- 1
H. Nakatsuji,
J. Ushio,
T. Yonezawa,
J. Organomet. Chem.
258
(1983) C1;
G. Schmid,
E. Welz,
Z. Naturforch.
B 34
(1979) 929;
H. Sakurai,
Y. Kamiyama,
Y. Nakadaira,
Angew. Chem.
90
(1978) 676;
10.1002/ange.19780900912 Google ScholarAngew. Chem. Int. Ed. Engl. 17 (1978) 674.
- 2 P. P. Gaspar in M. Jones, R. A. Moss (Eds.): Reactive Intermediates, Vol. 2, Wiley, New York, 1981, p. 335; R. S. Grev, H. F. Schaefer III, J. Am. Chem. Soc. 108 (1986) 5804: G. Raabe, H. Vančik, R. West, J. Michl. J. Am. Chem. Soc. 108 (1986) 671.
- 3 K. A. Brown-Wenseley, XIXth Organosilicon Symposium 1985, Louisiana State University, Baton Rouge, LA, USA.
- 4 H. Kang, D. B. Jacobson, S. K. Shin, J. L. Beauchamp, M. T. Bowers, J. Am. Chem. Soc. 108 (1986) 5668.
- 5 P. Jutzi, B. Hampel. K. Stroppel, C. Krüger, K. Angermund, P. Hefmann, Chem. Ber. 118 (1985) 2789; W. A. Herrmann, Angew. Chem. 98 (1986) 57; Angew. Chem. Int. Ed. Engl. 25 (1986) 56.
- 6 T. J. Marks. A. R. Newman. J. Am. Chem. Soc. 95 (1973) 769; D. Uhlig, H. Behrens, E. Lindner, Z. Anorg. Allg. Chem. 401 (1973) 233; M. D. Brice, F. A. Cotton, J. Am. Chem. Soc. 95 (1973) 4529.
- 7 D. H. Harris, M. F. Lappert, J. Chem. Soc. Chem. Commun., 1974, 859; P. J. Davidson, D. H. Harris, M. F. Lappert, J. Chem. Soc. Dalton Trans., 1976, 2268.
- 8 K. Farmery, M. Kilner, J. Chem. Soc., A 1970, 634.
- 9 Experimental procedure: A solution of 2 (5.0 g, 14.8 mmol) in 80 mL of THF was treated simultaneously with 1 (3.6 g, 14.8 mmol) in 20 mL of THF and Et3N (1.1 g, 14.8 mmol) in 10 mL of THF. After filtration al low temperature, the crude product was purified by column chromatography on a silica gel column cooled to −10°C (diameter 2.5 cm, height of silica gel 45 cm). Elution was carried out with THF, the quickly eluting, colorless zone being collected. After removal of the volatile products in vacuum, 3b was obtained as a colorless powder. Yield 0.76 g (12%); m.p. = 153 ° C. Addition of 0.5 mL of HMPT to the THF solution of 3b resulted in crystallization of 3a. Yield 0.96 g (12%), m.p. = 163 °C.–Compounds 3a, b could also be synthesized by treatment of a suspension of 1.7 g (5.0 mmol) of 4·0.5dioxane in 50 mL of THF at −40 °C with 1.2 g (5.0 mmol) of 1. The workup was the same as that described above. Yield 12%.
- 10 3a: 1H-NMR (60 MHz, C6D6): δ = 1.6 (s, 18H), 2.2 (d, 18H, 3J (31P1H) = 10.4 Hz); 13C-NMR (36.0 MHz, C7D8): δ = 31.9 (s, (H3C)3C), 36.1 (d, 2 J(31P13C) = 5.9 Hz, (H3C)2N), 72.5 (s, (H3C)3, C), 224.0 (s, COeq), 228.9 (s, COup); 29Si-NMR (53.54 MHz, C6D6): δ = 7.1 (d, 2 J (31P29Si) = 26.4 Hz); IR (KBr, cm−1) 2005 (w), 1920 (vs, br), 1883 (vs, br) ( vCO), 1000 (s) ( vPO), 633 (s) ( vPN). Correct elemental analyses (C, H, N, Si, Fe).–3b: 1H-NMR (60 MHz, C6D6): δ = 1.6 (s, 18 H), 1.8 (m, 4H), 3.6 (m, 4H; 13C-NMR (36.0 MHz, C7D8): δ = 32.0 (s, (H3C)3C), 71.9 (s, H3C)3 C), 223.5 (s, COeq), 229.1 (s. COap), 25.1, 68.5 (s, THF); 29Si-NMR (53.54 MHz, C6D6): δ = −9.4; MS (70 eV): m/z 414 ( M⊕, 7.3%). Correct elemental analyses (C, H, N, Si, Fe).
- 11 Crystal structure data: Enraf-Nonius CAD4 diffractometer, MoKα radiation, λ = 0.71069 Å, graphite monochromator, T = 23 °C. monoclinic, P21/n, a = 10.069(1), b = 16.950(1), c = 15.986(1) Å, β = 94.17(1)°, V = 2721.1 Å3, ρcalcd = 1.273 g cm−3, Z = 4, μ(MoKα) = 6.9 cm−1. 4761 unique reflections, 2812 with I ≥ 2.0 σ(I) “observed” (ϑ–2ϑ scan, Δρfin = 0.8+0.35 tan ϑ, hkl: +11, +20, ±18, (sinϑ/λ)max = 0.593). Lp, isotropic decay and empirical absorption correction: solution by direct methods (SHELXS-86), R = 0.054, Rw = 0.044, w = 1/σ2(F0) for 280 refined parameters (anisotropic, H constant, SHELXS-76); Δρfin(max./min.) = +0.50/−0.37 eÅ−3. Further details of the crystal structure investigation may be obtained from the Fachinformationszentrum Energie, Physik, Mathematik GmbH, D-7514 Eggenstein-Leopoldshafen 2 (FRG), on quoting the depository number CSD-52415, the names of the authors, and the journal citation.
- 12 A. R. Rossi, R. Hoffmann, Inorg. Chem. 14 (1975) 365.
- 13 Sixfold-coordinated Fe11 silyl compounds exhibit typical FeSi bond lengths of 2.22–2.45 Å, the shortest bonds being those to halosilyl ligands: M. Knorr, J. Müller, U. Schubert, Chem. Ber. 120 (1987) 879; L. Vancea, M. J. Bennett, C. E. Jones, R. A. Smith, W. A. Graham, Inorg. Chem. 16 (1977) 897, and references cited therein.
- 14
N. Wiberg,
G. Wagner,
G. Reber,
I. Riede,
G. Müller,
Organometallics
6
(1987) 35;
N. Wiberg,
K. Schurz,
G. Reber,
G. Müller,
J. Chem. Soc. Chem. Commun.,
1986 591;
G. Müller,
Nachr. Chem. Tech. Lab.
34
(1986) 778.
10.1002/nadc.19860340805 Google Scholar