Kinetic Relationships between Reactions in the Gas Phase and in Solution†
Dedicated to Professor W. Klemm on the occasion of his 70th birthday
Abstract
Some recent examples of reactions proceeding both in the gas phase and in solution have been investigated to determine their kinetics and mechanisms. The ratio of the corresponding rate constants, kG and kL, of the elementary processes studied has been found to be about unity for unimolecular reactions and between 1 and 10 for bimolecular reactions. The mechanisms, overall rates, rate constants, and activation energies have been determined for the homogeneous gas reaction NOCl + Cl2O = NO2Cl + Cl2 and the reaction NOCl + N2O5 = NO2Cl + 2 NO2, carried out in C2F3Cl3.



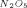 = 15.8 × 10−3 min−1 (40.5 °C), the value of kIV should be kIV = 7.9 × 10−3 min−1. The measurements on the systems ClO2/NO2/N2O4 and ClO2/N2O5 gave KI = 1.62 × 10−3 mm Hg−1 min−1 (40.5 °C) and kIV = 8.2 × 10−3 min−1 (40.5 °C) [1].
= 15.8 × 10−3 min−1 (40.5 °C), the value of kIV should be kIV = 7.9 × 10−3 min−1. The measurements on the systems ClO2/NO2/N2O4 and ClO2/N2O5 gave KI = 1.62 × 10−3 mm Hg−1 min−1 (40.5 °C) and kIV = 8.2 × 10−3 min−1 (40.5 °C) [1].

