3D-Printing Multi-Component Multi-Domain Supramolecular Gels with Differential Conductivity
Abstract
We report the use of wet-spinning to 3D-print gels from low-molecular-weight gelators (LMWGs) based on the 1,3 : 2,4-dibenzylidenesorbitol (DBS) scaffold. Gel stripes assembled from DBS-CONHNH2 and DBS-COOH are printed, and their conductivities assessed. Printed gels based on DBS-CONHNH2 can be loaded with Au(III), which is reduced in situ to form embedded gold nanoparticles (AuNPs). The conductivity of these gels increases because of electron transport mediated by the AuNPs, whereas the conductivity of DBS-COOH, which does not promote AuNP formation, remains lower. We then fabricate multi-component gel patterns comprised of spatially well-defined domains of printed DBS-CONHNH2/AuNP (higher conductivity) and DBS-COOH (lower conductivity) resulting in soft multi-domain materials with differential conductivity. Such materials have future prospects in applications such as soft nanoelectronics or tissue engineering.
Creating shaped and patterned conducting gels is a rapidly developing frontier of nanoscience that opens the prospect of next-generation soft nanoelectronic devices with a potential to interface with biological systems.1 Gels are composed of a ‘solid-like’ matrix that immobilizes a bulk liquid.2 Many gels used in everyday life are based on polymeric gelators (PGs), and indeed, PGs have been the primary focus in terms of developing conductive soft materials.3 However, there is increasing interest in supramolecular gels based on the self-assembly of low-molecular-weight gelators (LMWGs) into nanoscale structures, which form an extended network capable of immobilizing solvent.4 LMWGs have low cost, high synthetic tunability and potential environmental sustainability and biocompatibility, opening up high-tech applications.5 Increasingly, attention is focussed on shaping and patterning supramolecular gels to achieve sophisticated applications that rely upon spatially differentiated and potentially multivariate functionality.6
The 3D-printing of PGs has been widely explored with a view to enabling a variety of applications, including as conductive materials.7 In contrast, reports of the 3D-printing of LMWG-based supramolecular gels are more limited, owing to less established methodologies and the fact that relatively weaker supramolecular gels can be harder to physically manipulate.6 LMWGs have therefore not been exploited in 3D-printing conductive systems. To 3D-print LMWGs, extrusion-based methods in which the gel is extruded and then allowed to re-assemble in situ are the most widely explored. Adams and co-workers gained understanding of the impact of 3D-printing parameters on gelation outcomes,8 and attention has recently turned to extrusion-based printing multi-component systems, either via layer-by-layer printing or creating co-printed gels.9, 10 Combining LMWGs with other materials enables 3D-printing of soft composites with multifunctionality.[11]. For example, fluorescent additives have been incorporated into printed gels with potential relevance to soft photoelectronics.12 3D-printed LMWGs have also been used to support cell growth,13 including multi-component gels in which domains with different charges modified the cell growth morphology.14
An alternative to extrusion is wet-spinning. Early reports from the Stupp group demonstrated that injecting a LMWG dissolved in a good solvent into a coagulant solution gives rise to gel ‘noodles’.15 This approach was formalized by Fitremann and co-workers who optimized the wet-spinning of gel filaments (Figure 1B) and applied this approach to 3D-print larger objects (Figure 1C).16 Working with Fitremann, we demonstrated that our LMWGs based on 1,3 : 2,4-dibenzylidenesorbitol (DBS) were amenable to wet-spinning (Figure 1A), with 3D-printed objects based on DBS-CONHNH2 having excellent stability.17 Wet-spinning a mix of DBS-CONHNH2 and DBS-COOH led to co-assembly of the LMWGs into new nanostructures.18
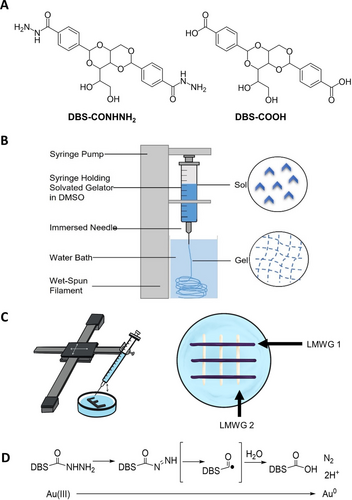
(A) Structures of low-molecular-weight gelators (LMWGs) DBS-CONHNH2 and DBS-COOH. (B) Schematic of wet-spinning method used for production of gel filaments. (C) Representation of the AxiDraw® wet-spinning set-up for 3D-printing macroscale gel patterns with physically distinct LMWG domains. (D) Mechanism of Au(III) reduction to Au(0) in situ by DBS-CONHNH2.
There has been interest in developing conductive materials based on LMWGs,5, 19 which can be formed by: (i) assembling gels in a conductive solvent (e.g., ionic liquids), (ii) designing LMWGs that assemble into inherently-conductive fibres, or (iii) loading conductive additives (e.g., nanoparticles, nanotubes, etc.) into a non-conductive gel.5c Generally, the conductivities of the resultant gels are tested in bulk, or as cast films. Surprisingly, although there are many 3D-printed conductive PGs,20 to the best of our knowledge, there have been no prior reports of 3D-printed conductive LMWGs, despite the promise of conductive hydrogels in sustainable energy technologies, regenerative medicine and bioelectronics.21 Furthermore, conductive soft materials are an integral part of biological systems — unique musculoskeletal tissues display diverse conductivities, and subtle differences in conductivity can be used as indicators to help diagnose a variety of pathologies.22 Progress to fabricate patterned soft supramolecular materials based on LMWGs, with differential conductivities in different parts of the printed pattern, would bring us a step closer to biomimicry and new technologies in soft bioelectronics. Herein, we report the 3D-printing of multi-component patterned LMWG gels with differential conductivities.
We used our established biocompatible DBS-CONHNH2 LMWG, which has a unique ability to reduce Au(III) to Au(0) and form gold nanoparticles (AuNPs) in situ within its gel matrix.17, 23 This reduction proceeds via oxidative conversion of the acylhydrazide group in the LMWG to a carboxylic acid (Figure 1D).24 Previously, we found that gels formed by conventional heat–cool methods displayed greater conductivity when loaded with AuNPs that could be augmented by partially dehydrating the gels to increase the local density of the conductive AuNP network, which we assigned to an electron hopping conductivity mechanism.23 Following this work, other researchers used our conductive AuNP-loaded gel to fabricate conductive glucose sensors.25 Using electrochemical impedance spectroscopy they reported a 10-fold decrease in resistance reflecting the increased electron transport rate. To achieve multi-component gel patterns with domains of differential conductivity, we therefore decided to 3D-print DBS-CONHNH2 gels alongside DBS-COOH gels that cannot support AuNP formation.
The LMWGs were synthesized according to our well-established methods26 and subjected to wet-spinning techniques as described by us in our previous collaborative work with Fitremann.17 We printed each LMWG by dissolving it in DMSO (1.5 % wt/vol) and injecting the solution into a water bath at a controlled flow rate (typically 6.8 μL/min) through a 23G needle (internal diameter=0.642 mm). The needle was moved in the x–y dimensions using an AxiDraw® machine (Figures 1C, S1) at a speed of 2.5 mm/s. As gel filaments formed upon injection into the water, they fell to the base of the petri dish with only a limited amount of spreading, allowing us to build up multiple layers and create pre-programmable shapes or patterns using the AxiDraw® interface. In a typical protocol, 16 layers were deposited on top of one another to create the final object. After printing every two layers, the needle was raised by 0.17 mm to avoid disrupting any printed layers and improve resolution. The objects printed with DBS-CONHNH2 and DBS-COOH at 1.5 % wt/vol LMWG loading were somewhat fragile, so the gels were reinforced by including 0.5 % wt/vol agarose PG — a well-known strategy27, 28 for preparing more robust LMWG-based gels that has not previously been applied in LMWG wet-spinning. The improved stability of the agarose-reinforced gels enabled us to subject printed objects to washing without significantly risking their physical integrity. In all cases, the 3D-printed wet-spun gels described from this point onwards also contain 0.5 % wt/vol agarose.
We explored ways to optimize AuNP loading into 3D-printed DBS-CONHNH2 gels by wet-spinning ‘stripes’ with a length of 2 cm and a width of ca. 3 mm (Figure 2A) as well as X-shaped patterns of similar dimensions (Figures S4–S6). Two processing methods were attempted: (Method I) printing DBS-CONHNH2 directly into AuCl3 solution and (Method II) incubating pre-printed DBS-CONHNH2 in AuCl3 solution. Using Method I, we found that during printing, AuNPs would form at the needle tip, initiated by DBS-CONHNH2, leading to blockages. Even after only printing the first two layers, gels would not print smoothly, resulting in unstable objects with poor resolution. The rapid kinetics of AuNP formation thus compete against the kinetics of injection and gel filament formation. We therefore switched to Method II. After printing, the water was carefully aspirated so as not to disturb the printed object and replaced with an AuCl3 solution. The DBS-CONHNH2 gels immediately adopted a deep purple colour, consistent with reduction of Au(III) and AuNP formation (Figure 2A, left), which developed further on incubating them for 24 h. Meanwhile, when exposing DBS-COOH gels to Au(III) in the same way, no purple colour was observed (Figure 2A, right), which is consistent with the active role of the acylhydrazide functional group in generating AuNPs.24
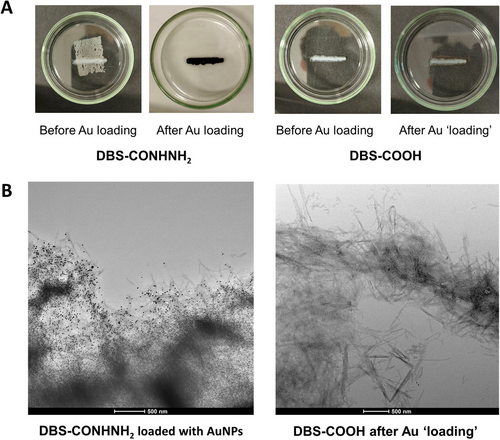
(A) Photographs of printed DBS-CONHNH2 and DBS-COOH stripes before and after gold loading. (B) TEM images taken of the ends of printed stripes after gold loading. Scale bar: 500 nm.
Scanning electron microscopy (SEM) confirmed the nanofibrillar nature of the printed gel stripes (Figures S23–S26). Transmission electron microscopy (TEM) supported the conclusion that AuNPs formed within DBS-CONHNH2 gels with mean particle diameters of ca. 20 nm (Figures 2B & S27–S28, S39) — the AuNPs fully perfuse the gel stripe. Ultimately the process of AuNP formation is a stoichiometric one,24 with the nanoscale fibrillar network limiting diffusion of the AuNPs once they reach a certain threshold size, preventing further aggregation. Analysis of TEM images from the end portion of the printed stripe gave an average AuNP diameter of 18.9±3.2 nm, while analysis of the central part gave a diameter of 21.2±4.2 nm, suggesting AuNPs grow slightly larger in the central part of the gel stripe where the local network density of DBS-CONHNH2 will be higher. Conversely, and importantly, for DBS-COOH there was no evidence of significant well-defined AuNP formation (Figures 2B & S29–S30, S40).
AuNP loading in 2-cm gel stripes was quantified by UV/Vis spectroscopy (Figures S7–S8; Table S1). For DBS-CONHNH2 exposed to 0.3 mM Au(III), the maximum uptake was 1400±150 mg/g, while using 0.4 mM Au(III), the maximum uptake was 1650±200 mg/g. In contrast, Au(III) uptake using DBS-COOH was significantly lower (257±50 mg/g at 0.3 mM). For DBS-COOH, the loading reflects passive diffusion of Au(III) ions into the gel and confirms that, unlike DBS-CONHNH2, there is no specific affinity/reaction between DBS-COOH and Au(III). UV/Vis analysis also confirmed that washing the AuNP-loaded objects once with deionized water was effective at removing the majority of excess Au(III), while leaving AuNPs intact (Figures S31–S34).
We then monitored the conductivity of 2-cm printed gel stripes loaded with different concentrations of Au(III) (Figures 3, S2–S3 & S9; Table S2) from end to end using the two-probe method with a digital conductivity meter and AC current. Baseline conductivities of DBS-CONHNH2 and DBS-COOH printed gels in the absence of AuNPs were found to be 14.4 and 27.8 μS/cm, respectively. We suggest the higher conductivity of DBS-COOH may reflect the potential of H+ to act as a charge transport element. However, both systems had low enough native conductivities for us to confidently distinguish the contribution of subsequently loaded AuNPs. Indeed, after incubating with 2 mM Au(III) and washing, the conductivity of the DBS-CONHNH2 gel increased to 90.2 μS/cm — a 6-fold increase (Figure 3B). This outcome is consistent with our previous work on bulk gels formed by heat–cool processing,23 where the conductivity of a hydrated DBS-CONHNH2 gel improved ca. 4-fold on Au(III) loading (enhanced to ca. 7-fold for the semi-dried gel). In contrast, after loading the DBS-COOH gel under the same conditions, only a 2-fold increase in conductivity (to 50.6 μS/cm) was observed relative to the unloaded gel, and significantly less than that of the AuNP-loaded DBS-CONHNH2 gel. These clear differences in conductivity between the two similarly treated gels highlight the role of AuNPs in enabling this property. As expected, AuNP-loaded DBS-CONHNH2 outperformed DBS-COOH at all Au(III) concentrations explored (Figure 3B).
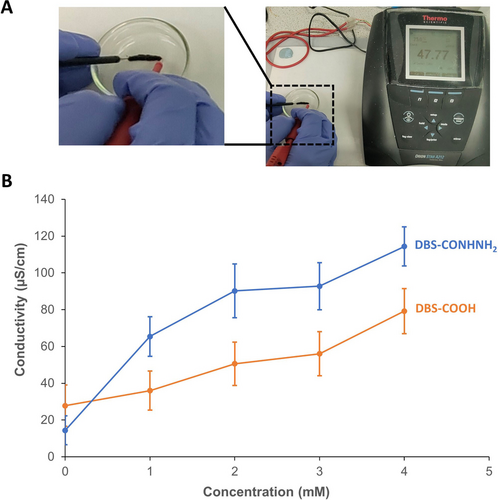
(A) Photograph of experimental set-up for measuring conductivity of printed gel stripes. (B) Conductivities of DBS-CONHNH2 (blue) and DBS-COOH (orange) after loading with Au(III) (0–4 mM). All gel stripes were washed once with water after Au(III) loading prior to measurement.
To understand the potential contribution of any residual Au(III) towards the small increase in conductivity for DBS-COOH gels, we subjected the gel objects to a second wash, which did reduce conductivities close to the baseline conductivity, i.e., 26.0 μS/cm for objects treated with 2 mM Au(III) (Table S3). On the other hand, DBS-CONHNH2 gels loaded with 2 mM Au(III) retained a significant conductivity of 60.0 μS/cm after two washes, remaining a factor of 4 above the unloaded DBS-CONHNH2 gel, indicating AuNP entrapment in this gel. These trends for both LMWGs were observed irrespective of the Au(III) concentration applied. However, subjecting printed gel objects to a second wash did come with the consequence of some mechanical damage, especially at higher AuNP loadings in DBS-CONHNH2. Therefore, we ultimately decided to continue our studies applying only a single washing step, although we note this will somewhat inflate the apparent conductivity of DBS-COOH as a result.
We then printed more complex patterns (Figure 4) of DBS-CONHNH2 and/or DBS-COOH to understand the impacts of patterning on conductivity. Initially, we printed X-shaped patterns (2-cm stripes of 16 layers each, one stripe printed directly over the other) based on a single LMWG to verify the influence of physical junctions on conductivity. Measuring conductivity between the four termini in pairs indicated that irrespective of the pathway, the object had a consistent overall conductivity (Figures S10–S12; Table S4). For example, after exposure to 2 mM AuCl3 and washing once with water, the conductivities of X-shaped DBS-CONHNH2 and DBS-COOH gels were 179.4 and 69.4 μS/cm, respectively (Figure 4A). Once again, the AuNPs in the DBS-CONHNH2 gel enhance the conductivity (12.5-fold) in contrast to that of DBS-COOH (2.5-fold). The conductivities observed for the X-shaped objects were higher than those for single stripe objects, which we attribute to the extra thick 32-layer crossover junction.

Photographs of 3D-printed X-shaped gel patterns and conductivities as a function of Au(III) concentration. (A) Gels printed in isolation, i.e., in separate petri dishes, and loaded with Au(III); DBS-CONHNH2 (blue), DBS-COOH (orange). (B) Gels printed together, i.e. in the same petri dish, and simultaneously loaded with Au(III); DBS-CONHNH2 (blue), DBS-COOH (orange).
We then printed multi-component X-shaped patterns of similar dimensions with one stripe consisting of DBS-CONHNH2 and the other of DBS-COOH (Figures 4B & S13–S14) to examine the potential for differential conductivity. The DBS-CONHNH2 and DBS-COOH stripes were printed sequentially into water, the water exchanged with AuCl3 solution, and the gel objects washed once with water after incubation. Surprisingly, we observed the same conductivity irrespective of electrode placement or domain involved (Figures 4B & S15; Tables S5–S6). Both gel stripes had similar conductivities of ca. 80 μS/cm at 2 mM Au(III) loading. In addition to the expected intense purple colour of the DBS-CONHNH2 component, the DBS-COOH stripe now adopted a pale purple colour. This outcome was not observed for DBS-COOH gels printed in isolation, which remained colourless on exposure to Au(III). We wondered whether the order of printing (DBS-CONHNH2 first) meant some trace DBS-CONHNH2 LMWG remained dispersed in the water bath and became incorporated at low levels into the DBS-COOH stripe during printing, enabling AuNP formation. We tested this hypothesis by switching the order of printing, starting with DBS-COOH, followed by DBS-CONHNH2, but similar colour uptake and conductivities were observed, suggesting residual DBS-CONHNH2 incorporation during DBS-COOH assembly is highly unlikely.
In an attempt to gain clarity on this observation, we decided to once again wet-spin each LMWG in the same petri dish, but as parallel stripes, i.e., with no contact points (Figures 5 and S15), to understand if the physical junction was facilitating AuNP loading. To our surprise, upon exposure to Au(III) solution, in addition to the expected intense purple colour in the DBS-CONHNH2 stripe, a pale purple colour associated with AuNP loading was still observed in the DBS-COOH stripe (Figure 5A), the intensity of which depended on the Au(III) concentration used. TEM (Figures S35–S38 and S41–S42) confirmed AuNPs with diameters of 10–30 nm were incorporated into the DBS-COOH gel. Due to the lack of a physical junction, we propose that the transfer of smaller AuNPs pre-formed by the DBS-CONHNH2 stripe to the DBS-COOH gel stripe can take place without even requiring physical gel–gel contact. Diffusion seems to occur through the intervening solution from one gel domain to another. We suspect that such diffusion may be more favoured for smaller AuNPs — indeed the AuNPs observed in the end portion of DBS-COOH in this experiment had a mean diameter of 18.2±4.2 nm, which was slightly smaller than those left in the end portion of DBS-CONHNH2 (mean diameter of 22.6±5.1 nm in this experiment), which is also slightly larger than when a single DBS-CONHNH2 stripe was loaded with AuNPs (see above). This finding provides new and unexpected insights into the formation of precious metal NPs in these gels and highlights the dynamic nature of the Au reduction process. As before, the conductivities of the two independently printed gel stripes were similar (Figure 5B; Table S7), though we note that a slight relative increase in conductivity of the DBS-CONHNH2 stripe was observed over the DBS-COOH one. The unexpected degree of ‘communication’ between physically separate gel objects discovered here offers a potentially intriguing way of tuning the differential properties of multidomain soft nanomaterials. Though beyond the scope of this current study, such processes may ultimately lead us closer to developing dynamic electronic processes relevant to biological systems.22
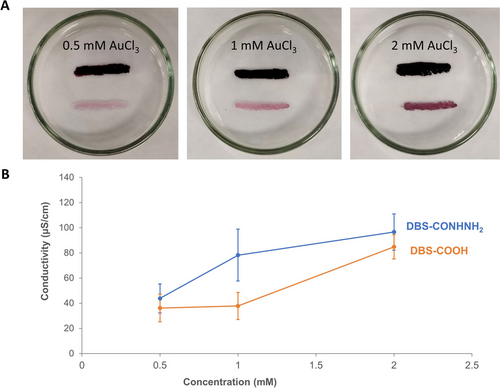
(A) Photographs of 2-cm parallel gel stripes of DBS-CONHNH2 (upper) and DBS-COOH (lower) treated simultaneously with Au(III) solutions at different concentrations. (B) Conductivities of the gel patterns shown in (A) as a function of Au(III) concentration, with the DBS-CONHNH2 component (blue) and DBS-COOH component (orange), having broadly similar conductivities.
Taking all these observations together, we finally developed a stepwise 3D-printing protocol to fabricate multi-component multi-domain gel patterns with distinct conductivities based on the two LMWGs. Thus, our preferred approach was as follows and as shown in Figure 6A: (i) the DBS-CONHNH2 component was wet-spun as a 2-cm stripe (16 layers) into water and incubated in a Au(III) solution for 24 h before (ii) the Au(III) solution was exchanged for water, (iii) the DBS-COOH stripe was printed into the water, then (iv) the gels were washed once with water (Figures S16 & S19). This protocol ensured that the DBS-CONHNH2 domains were exclusively loaded with AuNPs, as indicated by its deep purple colour, in contrast to the DBS-COOH domains, which remained colourless and AuNP-free (Figures 6B, S17 & S20). Furthermore, the step to replace the Au(III) solution with water could also be omitted: printing DBS-COOH directly into the used Au(III) solution also maintained orthogonal (differential) AuNP loading.
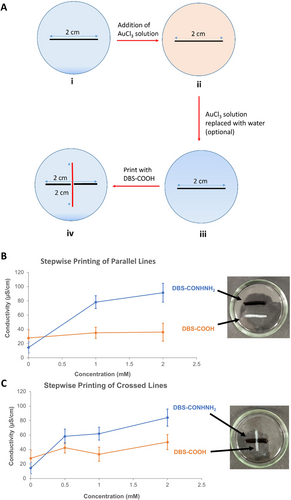
(A) Stepwise approach to printing of an X-shaped pattern with comprising AuNP-loaded DBS-CONHNH2 (black) and DBS-COOH (red) printed sequentially, (B) Differential conductivities and photographs (1 mM AuCl3) of multi-component gel patterns comprising AuNP-loaded DBS-CONHNH2 (blue) and DBS-COOH (orange) printed sequentially a pattern of parallel 2-cm stripes, and (C) Differential conductivities and photographs (1 mM AuCl3) of a 2-cm×2-cm X-shaped pattern of a DBS-COOH component printed over an AuNP-loaded DBS-CONHNH2 stripe.
Pleasingly, significant differential conductivity was achieved between the LMWG domains. In the case of parallel stripes (Figure 6B), the DBS-CONHNH2 domain exhibited a 6-fold increase in conductivity of 91.4 μS/cm with respect to its baseline conductivity when loaded with 2 mM Au(III). Meanwhile, the conductivity of the DBS-COOH domain remained low and approximately constant irrespective of the Au(III) concentration employed (Figures 6B & S18, Tables S8 & S9). These results support the potential of each LMWG to affect the differential conductivity of multi-component gels as a consequence of their molecular-scale programming.
We finally applied this stepwise protocol to 3D-print X-shaped patterns featuring a physical junction between a 2-cm DBS-COOH stripe overlaying a DBS-CONHNH2 one (Figures 6C & S21–S22). The DBS-COOH domain remained colourless, with no evidence of AuNP loading, despite the direct physical contact between the two gel components. To our delight, the conductivity of the stripes was significantly different, i.e., 6-fold greater in the DBS-CONHNH2 domain compared to baseline when subjected to 2 mM Au(III). The conductivity of the DBS-COOH domain remained relatively unchanged (Fig, 6C; Table S10). We were able to tune the extent of differential conductivity by controlling the concentration of Au(III) used to pre-load the DBS-CONHNH2 component, demonstrating that multi-component multi-domain hydrogels can be 3D-printed to display tunable differential conductivity pathways. This work constitutes an important first step towards 3D-printing soft biocompatible electronic devices with well-defined conducting pathways from easily synthesized, low-cost, readily processable supramolecular building blocks.
In conclusion, we successfully fabricated 3D-printed multi-component multi-domain soft materials based on two LMWGs by wet-spinning in which the conductivity of one component (DBS-CONHNH2) is selectively amplified by loading with AuNPs vs a less conductive component (DBS-COOH). Specifically, AuNPs can increase the conductivity of DBS-CONHNH2 up to ca. 12.5-fold, depending on the amount of gold loaded into the gel. Loading printed gel patterns containing both DBS-CONHNH2 and DBS-COOH provides new insight into AuNP formation, with AuNPs appearing to diffuse from one gel to another to induce conductive properties in an otherwise less-conductive gel. Ultimately, by fabricating 3D-printed patterns in a stepwise manner, we created designs in which different pathways can possess different conductivities as a result of their molecular-scale programming.
Although conductivity in supramolecular gels is a known phenomenon, combining this with the ability to shape and pattern “on demand” and in programmable ways opens up new horizons for the development of advanced soft materials, particularly given the biocompatible nature of the LMWGs.17 As a limitation, we note that in absolute terms, the conductivities of even the AuNP-loaded DBS-CONHNH2 gels remain relatively low (ca. 100 μS/cm). However, by developing 3D-printed supramolecular gels with greater absolute conductivities, we are optimistic that approaches such as that reported herein to print patterned gels with distinct conducting and insulating domains could one-day enable the realization of soft biointerfaced nanoelectronic circuitry or electro-responsive soft materials for the differential stimulation of stem cells for precise tissue engineering. Research towards these targets is ongoing in our laboratories.
Acknowledgments
We thank the University of York Chemistry Wild Fund and Dr Tony Wild for supporting this research through the award of a PhD scholarship (T.T.V). A.-J.A. gratefully acknowledges The Royal Society for their support with a Dorothy Hodgkin Research Fellowship (DHF\R1\180106).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




