Metal–Organic Frameworks Derived Carbon-Supported Metal Electrocatalysts for Energy-Related Reduction Reactions
Abstract
Electrochemical reduction reactions, as cathodic processes in many energy-related devices, significantly impact the overall efficiency determined mainly by the performance of electrocatalysts. Metal–organic frameworks (MOFs) derived carbon-supported metal materials have become one of star electrocatalysts due to their tunable structure and composition through ligand design and metal screening. However, for different electroreduction reactions, the required active metal species vary in phase component, electronic state, and catalytic center configuration, hence requiring effective customization. From this perspective, this review comprehensively analyzes the structural design principles, metal loading strategies, practical electroreduction performance, and complex catalytic mechanisms, thereby providing insights and guidance for the future rational design of such electroreduction catalysts.
1 Introduction
The use of fossil fuels remains the foundation of current industrial system, accounting for approximately 80 % of global primary energy demand.1 However, the inherent nature of fossil fuels as non-renewable resources renders them incapable of serving as the core energy source for sustainable human development.2 Furthermore, the extensive emission of carbon/nitrogen compounds resulting from human production, daily activities, and industrial operations, has significantly worsen the environment.3 The trends of increasing pollution and climate change impose enormous burdens on the development of human society.4 Electrochemical technologies provide a viable solution to address the energy and environmental crises. Currently, with the significant reduction in the costs of renewable energy such as photovoltaic, wind, and hydroelectric power, utilizing surplus renewable electricity to drive various electrolytic reactions can yield cleaner energy forms or transform pollutants into value-added chemicals.
In the renewable electricity-driven electrocatalytic landscape, electrochemical means concerning artificial hydrogen, carbon, and nitrogen cycles have emerged as particularly significant approaches (Figure 1).5 The artificial hydrogen cycle involves the coupling of hydrogen production via water electrolysis and hydrogen utilization via fuel cells, enabling the hydrogen-water cyclic utilization.6 The artificial carbon cycle focuses on the core technology of electrocatalytic carbon dioxide (CO2) conversion, transforming CO2 into valuable small molecules (such as gaseous CO, CH4; liquid C1 and Cn(n≥2) products).7 And the artificial nitrogen cycle primarily involves the electrochemical conversion of nitrogen gas (N2) and nitrogen oxides (NO, NOx) into nitrogen-containing molecules such as ammonia (NH3), hydroxylamine (NH2OH), and hydrazine (N2H4).8 These man-made interventions will provide a boost to the earth's overall chemical cycle, thereby relieving the dual pressures of energy and environment. Electrocatalysts play an indispensable role in the aforementioned electrochemical technologies. Under an applied electric field, they provide alternative reaction pathways with lower activation energies, accelerating reaction rates and reducing the energy consumption of electrochemical devices.9 Currently, metal-based catalysts are widely utilized in these electrochemical reactions, with their activity and stability being the two core assessment parameters.10 The development of electrocatalysts that possess excellent catalytic activity and durability is a crucial frontier in the field of electrocatalysis.
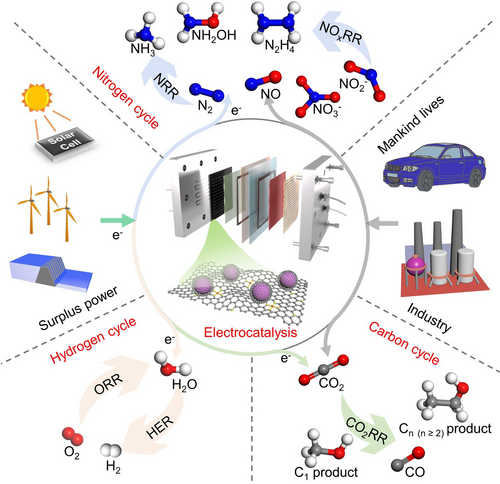
Schematic of renewable electricity-driven electrocatalytic landscape mainly consisting of artificial hydrogen cycle, carbon cycle, and nitrogen cycle.
Metal–organic frameworks (MOFs) contain metal atoms or clusters as nodes, which are coordinated with organic ligands to form diverse structures.11 Due to the high tunability of metal nodes and organic ligands, MOFs exhibit various compositions and morphologies. Additionally, the introduction of different modifiers (such as surfactants, small molecules, templates) further allows for the adjustment of their component and structure (Figure 2).12 However, owing to the poor conductivity of most MOFs, they are not suitable for direct use as electrocatalysts.13 Therefore, through the pyrolysis process in the protective atmosphere, organic ligands are thermally decomposed into heteroatom-doped carbons, significantly enhancing the overall conductivity. Simultaneously, the metal species are transformed into single atoms, clusters, and/or nanoparticles, eventually resulting in various MOF-derived carbon-supported metal catalysts.14 These catalysts own several advantages: 1) the multiple selection of different metals and ligands allows for a wide range of compositions and morphologies, providing numerous catalyst candidates for different catalytic reactions; 2) the inherited hierarchical porous structure ensures sufficient exposure of catalytic active centers, as well as rapid mass and electron transfer processes; 3) the strong metal-support interaction (SMSI) enables the electronic structure modulation of active species, and the provision of armor-like protection effect.15 Compared with pristine MOFs, especially two-dimensional conductive MOFs, MOF-derived catalysts have certain superiorities in ligand selectivity, electrical conductivity, tunable catalytic center configuration, and structural stability with carbon coating.16 It should be noted that these MOF-derived catalysts are always more suitable for electrochemical reduction reactions (mainly including hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), carbon dioxide reduction reaction (CO2RR), nitrogen reduction reaction (NRR), and nitrogen oxides reduction reaction (NOxRR)), rather than oxidation reactions. This is because MOF-derived carbon-supported metal catalysts can well maintain relatively stable structures and phases at the reduction potentials and be exempt from serious carbon substrate oxidation, thus ensuring long-term stability.17
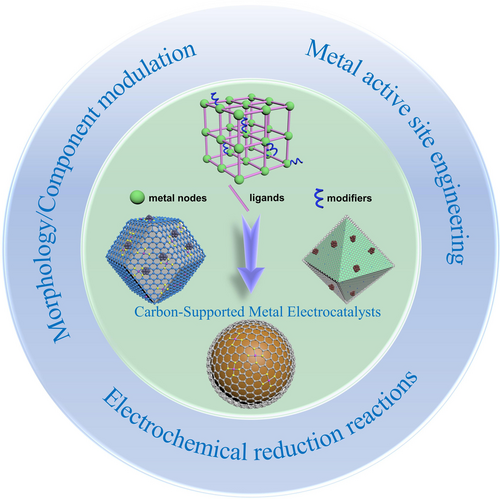
Illustration of MOF-derived carbon-supported metal electrocatalysts towards electrochemical reduction reactions.
Herein, the influences of ligands and modifiers on the morphology, composition, and defect structure of MOF-derived carbon frameworks are first systematically summarized. Modulation in the morphology dimension relies on the selection of ligands, modifiers, syntheticism, and post-processing means. And the elaborate regulation of heteroatom dopants and pore structures offers the necessary microenvironment for effective anchoring of active metal species. Furthermore, we delve into the controlled metal loading methods, including original metal node selection, metal ion exchange, spatial confinement strategy, and post-processed directed assembly. MOF-derived carbons serve as platforms for a wide variety of metallic single atoms/dimers, nanoclusters, and nanoparticles. Subsequently, the applications of MOF-derived carbon-supported metal catalysts in electrochemical reduction reactions over the past few years are retrospectively reviewed, from the perspectives of structural morphology, active center analysis, and structure–activity relationship. Finally, thorough challenge analyses and prospective outlooks regarding the applications of such catalysts in electrochemical reduction reactions are conducted. Although some reviews have summarized the application of MOF-derived catalysts in various electrocatalytic reactions, they have not well analyzed the underlying principles of morphology and composition regulation, as well as their widespread applications in electroreduction reactions.18 Thus, in this review, the action mechanism of three key precursor elements, namely metal nodes, ligands, and modifiers, on the morphology/component of pristine MOFs and their derivatives has been comprehensively analyzed. Applications of MOF-derived carbon-supported metal catalysts in diversified electroreduction reactions have been teased out, hoping to provide some constructive guidance for the rational construction of such catalysts.
2 Ligand and Modifier Regulation for Carbon Matrixes
Various factors in the synthesis process of MOFs can potentially influence the final product's morphology, including metal salts, organic ligands, modifiers, solvents, and reaction conditions.19 Unlike other factors that rely more on trial-and-error synthesis, the selection of ligands and modifiers has a more traceable impact on MOFs and their derived carbon matrixes. By utilizing specific organic ligands and modifiers, the control-oriented self-assembly of MOF structures can be achieved, thereby obtaining carbon matrixes with different dimensions, compositions, and defect structures.20 Thus, this section would primarily introduce the regulatory effects of ligands and modifiers on the morphology, component, and configuration of carbon frameworks, while also interspersing some important auxiliary approaches.
2.1 Morphology Control
The utilization of different metals and/or ligands during the synthesis process can alter their mutual connectivity, resulting in the formation of distinct structures. This is primarily attributed to the varying sizes and shapes of metal nodes and organic ligands, which affect the number and direction of bonds.21 It has been reported that the structure and morphology of MOFs can be controlled by adjusting: 1) the number of metal atoms in the secondary building units (SBUs); 2) the number of ligands; and 3) the number of SBUs coordinated with a single ligand.22 More complex MOF structures can be also achieved by mixing different SBUs and ligands. Many MOFs, especially zeolitic imidazolate frameworks (ZIFs), can well retain their original morphology after one-step or stepwise pyrolysis. However, the structure of some MOFs will be partially collapsed or even completely destroyed after the high-temperature pyrolysis. This is related greatly to the thermal stability of MOFs. For these MOFs with poor structural stability at high temperatures, the original morphology and porosity could be better maintained by pyrolysis at low temperatures. But this also leads to the more pronounced amorphous characteristics of carbon matrix, resulting in downsides such as low electrical conductivity. The compromise between morphology and conductivity requires more systematic investigations on pyrolysis conditions. Based on the dimensional differences of the resulting carbon-based frameworks, they can be primarily classified into one-dimensional, two-dimensional, and three-dimensional MOF-derived carbons.
2.1.1 One-Dimensional (1D) MOF-Derived Carbon
1D MOF-derived carbons refer to carbon nanomaterials with distinct linear morphologies and high aspect ratios of length to diameter (Figure 3A). They primarily include carbon nanofibers, nanorods, nanotubes, etc.23 As 1D radial nanosized carbons, these materials exhibit notable mechanical, photoelectrochemical, and other properties.24
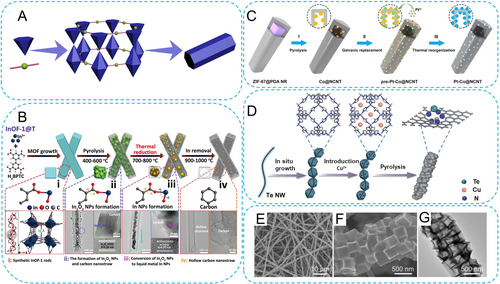
(A) Topological driving force for 1D MOFs. (B) Synthetic method of InOF-1 derived 1D hollow carbon nanostraw. Reproduced with permission.25 Copyright 2022, Wiley-VCH. (C) Schematic of the synthesis of Pt−Co@NCNT. Reproduced with permission.29 Copyright 2021, Wiley-VCH. (D) Synthetic method of TeN2-CuN3 DAC. Reproduced with permission.32 Copyright 2023, Nature Publishing Group. (E, F) Scanning electron microscope (SEM) and (G) TEM images of Cu/Zn-N/P-CMF. Reproduced with permission.36 Copyright 2023, American Chemical Society.
First, the choice of ligands is the most direct factor influencing the formation of rod-like structures. Chai et al. utilized biphenyl-3,3′,5,5′-tetracarboxylic acid (H4BPTC) as the organic ligand, which was coordinated with indium salt to form InOF-1 rods during a solvothermal reaction (Figure 3B).25 This In-based MOF belongs to the tetragonal crystal system, whose nanochannels are formed by In−O bonds with the 6-coordinate environment. The selection of this ligand determines the preferential growth along the longitudinal direction, therefore obtaining rod-like structures. The rod morphology was preserved after the subsequent heat treatment under an inert atmosphere. During this process, the metal species underwent the transformation from In2O3 to In, and finally completely evaporated, while the as-derived carbon gradually formed a hollow nanostructure. Besides, fumaric acid is another organic ligand that readily forms rod-like structures. Our group demonstrated uniform hexagonal rod-like MIL-88A (MIL stands for Materials from Institute Lavoisier) solid nanostructures by hydrothermal synthesis using fumaric acid and iron/nickel salts.26 As the doping amount of Ni increases, the length-to-diameter ratio gradually decreases. Furthermore, through phytic acid etching and subsequent heat treatment, Ni-doped FeP/C hollow nanorods can be conveniently obtained. In addition, MIL-53 is a type of MOF material formed by 1,4-benzenedicarboxylate (1,4-BDC) and FeO6 octahedra, possessing one-dimensional mass transport channels. The FeO6 units can also be replaced by NiO6 units. Li et al. combined 1,4-benzenedicarboxylate (1,4-BDC) with nickel and iron salts to construct Ni-MIL-53 nanorods, whose length-to-diameter ratio was approximately nine.27
In addition to ligand selection, the introduction of surfactants can serve as effective modulators to control the nucleation and oriented growth processes of MOFs, thus obtaining 1D structures. Cetyltrimethylammonium bromide (CTAB) is one of the most typical surfactants, and it can significantly reduce the growth rate of [100] facets in ZIFs, thereby altering their morphology.28 Zhang et al. introduced a large amount of CTAB into the synthesis system of ZIF-67, thereby obtaining ZIF-67 nanorods through hydrothermal reaction.29 Subsequently, polydopamine (PDA) was coated onto the surface of ZIF-67 nanorods. The thermal decomposition resulted in Co-incorporated N-doped carbon nanotubes. Through galvanic replacement and thermal reorganization, highly efficient Pt-Co@NCNT catalysts could be prepared (Figure 3C). Furthermore, salicylic acid has also been reported as an effective modulator capable of regulating the morphological growth of MOFs during hydrothermal processes.30 The addition of salicylic acid modulator effectively stabilizes the surface active metal sites of MOF-74 crystals, promoting the growth of MOF in a rod-like architecture.
Apart from the above template-free approach of directly changing the types of ligands and modifiers, the utilization of 1D templates allows for the formation of carbon-based composite materials with unique 1D structures as well. Tellurium (Te) nanowires serve as self-sacrificing templates for the growth of various MOF materials on their surfaces, which can be removed during the pyrolysis process.31 As shown in Figure 3D, the ZIF-8 layer was densely grown on the surface of Te nanowires, forming a core-sheath structure. Through Cu2+ impregnation treatment and subsequent pyrolysis, non-planar and asymmetric diatomic TeN2-CuN3 sites were obtained.32 Furthermore, typical carbon nanotubes (CNTs) can also be utilized as 1D templates. Through self-assembly of Co2+, PMo12O403−, and 2-methylimidazole on the surface of CNTs, polyoxometalates (POMs) were encapsulated within the pores of ZIF-67, while ZIF-67 could be interconnected along the CNTs, forming a 1D composite material.33 Upon high-temperature pyrolysis, Mo-doped Co3O4 supported on CNTs could be obtained. In addition to inorganic templates, 1D polymer fibers can also serve as template agents. Our group first constructed polyacrylonitrile/cobalt acetate (PAN/Co(Ac)2) composite fibers, which were then coordinated with 2-methylimidazole to form a dense ZIF-67 shell on their surfaces as the core components. By removing the internal PAN template, 1D hollow fibrous structures composed of stacked ZIF-67 units could be obtained.34
Besides, electrospinning could also be utilized to fabricate 1D fibrous structures, making it compatible with MOFs to directly construct composite materials with 1D features.35 For example, Zeng et al. recently conducted phytic acid etching and Cu ion exchange on ZIF-8 nanocubes, followed by blending them with PAN to prepare a composite slurry.36 Through electrospinning, PA-ZnCu@PAN fibers were obtained. Subsequent stepwise thermal treatment and acid etching resulted in the formation of N, P-codoped carbon macroporous fibers with isolated Zn/Cu atomic distribution (Figure 3E–G). A similar report demonstrated the co-electrospinning process of Mn-BTC nanospheres with PAN, resulting in a fibrous composite with the 1D beads-in-string structure.37 By adjusting the mass ratio of Mn-BTC nanospheres to PAN, a necklace-like structure with separated Mn-BTC spheres in the fiber strings or a botryoid structure with Mn-BTC spheres clustered along the fiber strings could be obtained, respectively. Through pre-oxidation and high-temperature pyrolysis, yolk-shell MnOx nanoparticles embedded in the 1D N-doped carbon nanofibers were ultimately prepared.
To sum up, only certain ligands and cooperative metal ions can be utilized to construct 1D MOF-derived structures. In general, these ligands can only play a role in directionally 1D-induced growth under certain reaction conditions. Using modifiers to restrict the growth of specific crystal faces also puts harsh requirements on the types and growth conditions of MOFs. These two methods are of good exploratory significance from the viewpoint of chemical synthesis, but significantly limit the types of MOFs that can be used. By contrast, by means of 1D templates or electrospinning technologies, almost all MOFs can theoretically be selected, which will greatly increase the number of possible MOFs. In any case, these 1D MOF-derived carbons, comprising multiple morphologies and components, continue to be extensively investigated as significant derivatives.
2.1.2 Two-Dimensional (2D) MOF-Derived Carbon
2D carbon nanomaterials refer to structures that grow freely in the a and b directions, while being limited to a few layers or even monolayer thickness in the c direction (Figure 4A).15a Well-defined 2D MOF nanostructures and their derived carbon-based frameworks can be obtained through bottom-up or top-down synthetic methods. These 2D MOF-derived carbons normally exhibit high specific surface areas and excellent electronic, mechanical, and thermal conductivity properties, making them widely applicable in the energy field.38
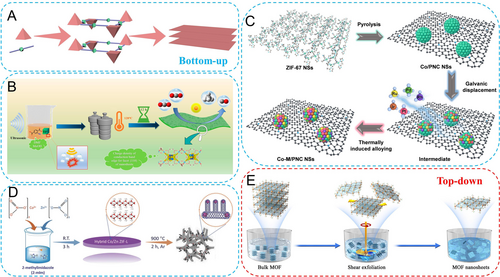
(A) Topological driving force for 2D MOFs. (B) Schematic of the construction of ultrathin NH2-MIL-125 NSs. Reproduced with permission.40 Copyright 2022, American Chemical Society. (C) Illustration of the synthetic method for Co−M alloy/PNC NSs. Reproduced with permission.42 Copyright 2023, Wiley-VCH. (D) Schematic illustration of synthetic process for Co-N-CNTs. Reproduced with permission.46 Copyright 2018, Wiley-VCH. (E) Shear exfoliation process of ELM-12 nanosheets. Reproduced with permission.48 Copyright 2020, American Chemical Society.
The bottom-up strategies refer to the controlled growth of the coordinated structure in a specific direction during the MOF synthesis. The anisotropic growth of MOF crystals leads to a significantly higher growth rate on the high-energy horizontal facets than that on the low-energy vertical facets.39 Such a target necessitates the selection of appropriate ligands. Guo et al. employed 2-amino-1,4-benzenedicarboxylic acid as the organic ligand, which coordinated with tetrabutyl orthotitanate in a mixed solvent, resulting in the formation of NH2-MIL-125 nanosheets with the maximum exposed [110] facet (Figure 4B).40 The exposed dominated [110] facets were directly observed by transmission electron microscope (TEM), while the diffraction intensities of (110) and (220) planes were significantly enhanced in the X-ray diffraction (XRD) pattern. Such pronounced growth on the high-energy facets led to a 2D MOF with a thickness of ~12 nm, facilitating the full exposure of active centers.
The introduction of surfactants as capping agents is another important strategy for obtaining 2D MOFs. Sodium dodecyl sulfate (SDS) has been demonstrated to own the most stable adsorption on the (002) facet of ZIF-67 and effectively reduce the exposure energy barrier of this crystal plane, thus obtaining thin-layered nanosheet structures with exposed (002) facets.41 Qiu et al. introduced SDS as a modifier in the synthesis of ZIF-67, leading to the fabrication of 2D ZIF-67 nanosheets (Figure 4C).42 Through the sequential pyrolysis-galvanic displacement-thermal reduction process, Co−M alloy catalysts (M=Pt group metals) supported on porous NC nanosheets were obtained. Similarly, polyvinylpyrrolidone (PVP), as an amphiphilic polymer surfactant, can induce the anisotropic growth of MOFs into 2D nanostructures. Shi′s group utilized the carbonyl (C=O) groups in PVP molecules to axially coordinate with two Zn atoms in Zn2(COO)4 units.43 Ultrathin interlayers were formed between the planar coordination structures, thereby inducing the formation of 2D Zn-MnTCPP-PVP nanosheets.
It should be noted that the choice of solvent can also have a certain influence on the microscale coordination of MOFs, thereby providing possibilities for obtaining 2D MOFs.44 A typical example is the formation of rhombic dodecahedron structure ([Zn(mim)2] (mim=2-methylimidazolate)) in the traditional methanol system, while leaf-like ZIF-L structure (Zn(mim)2 ⋅ (Hmim)1/2 ⋅ (H2O)3/2 or C10H16N5O3/2Zn) (Hmim=2-methylimidazole) can be formed in the water system.45 ZIF-L is a typical 2D layered structure composed of two crystallographically different Zn ions and fully bridged/monodentate/free Hmim molecules. Wang and co-workers prepared bimetallic Co/Zn ZIF-L nanosheets in the water system (Figure 4D).46 During the subsequent pyrolysis process, Zn atoms were evaporated, and Co atoms were gradually reduced to nanoparticles which induced the growth of carbon nanotubes.
The top-down strategies are to strip layered MOF solids into 2D MOF nanosheets by physical or chemical exfoliation. Such strategies are particularly suitable for MOF solids with weak interlayer interactions (e.g. hydrogen bonding, van der Waals forces, π–π stacking).15a Ultrasonic stripping is the most typical physical stripping approach that can effectively overcome the interlayer weak forces without disrupting the covalent bonds within each layer. Liu et al. acquired the ultrathin MOF nanosheets by ultrasonic exfoliation of Zn2(bim)4 (bim=benzimidazole) precursors with the assistance of ionic liquids.47 Moreover, shear stripping is another important physical stripping method. Shearing forces generated by commercial blenders can provide high-yield production for large-scale commercial applications. As shown in Figure 4E, under the shear forces, bulk ELM-12 (Cu(4,4-bipyridine)2 ⋅ (trifluorosulfonic acid)2) and Zn2(BIM)4 can both be peeled into thin nanosheets with a thickness of only 3–5 nm.48 Differently, chemical stripping methods rely on chemical reactions to break the interlayer coordination bonds and obtain stable 2D MOF structures. Ding et al. used a chemically labile intercalating agent, 4,4′-dipyridyl disulfide (DPDS), to chemically intercalate Zn2(Pd-Tetrakis(4-carboxyphenyl)porphyrin), forming a novel intercalated MOF.49 The disulfide bond of the intercalating agent was then chemically reduced using trimethylphosphine. Thus, the interlayer force was weakened and the interlayer spacing was expanded, thereby facilitating the delamination into ultrathin nanosheets with a thickness of approximately 1 nm.
In general, bottom-up methods are more direct and controllable, but they tend to be more complex in terms of synthesis. While top-down strategies offer simpler operations but typically result in lower yields. Therefore, developing more rational synthetic strategies to enable the convenient preparation of 2D MOF-derived carbons is still urgent.
2.1.3 Three-Dimensional (3D) MOF-Derived Carbon
The bonds between metal nodes and ligands have high quantities and diverse orientations, and anisotropic growth rates of different directions for most MOFs are not significantly different. Thus, they tend to assemble into 3D structures with multiple exposed crystal facets (Figure 5A).50 Moreover, owing to the stable coordination structure, the original morphology of 3D MOFs can be easily preserved during the thermal decomposition process. Although the 3D MOF-derived carbons are seemingly solid, they often own high surface area and well-developed pore structures resulting from the retention of original porosity from MOFs.
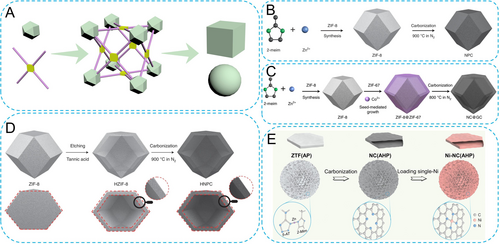
(A) Topological driving force for 3D MOFs. Schematic illustration of synthetic processes for (B) solid NPC, (C) core–shell NC@GC and (D) hollow HNPC. Reproduced with permission.51 Copyright 2022, Nature Publishing Group. (E) Schematic of the preparation process for Ni-NC(AHP). Reproduced with permission.55 Copyright 2022, Wiley-VCH.
Solid polyhedrons are the most common 3D MOF-derived carbon structures. As shown in Figure 5B, ZIF-8 was obtained by coordinating Zn ions with 2-methylimidazole in a methanol solvent. With temperature increasing under an inert atmosphere, Zn species were first reduced to nanoparticles and then gradually evaporated, resulting in the formation of porous N-doped carbon nanomaterials.51 Such 3D carbon-based frameworks provide efficient platforms for supporting metallic active species, which will be discussed in the next section. Besides these ZIFs, various types of MOFs, such as UiOs52 and MILs,53 can be also thermally decomposed into stable 3D carbon networks.
Except for the solid polyhedrons, researchers have also developed other 3D MOF-derived carbons with various special architectures. As shown in Figure 5C, further introducing Co salts into ZIF-8 dispersion can induce the surface growth of ZIF-67 layers, forming core–shell ZIF-8@ZIF-67.51 Subsequent carbonization process results in nitrogen-doped carbon@graphitic carbon (NC@GC). Similarly, by in situ growth of ZIF-67 layers onto the surface of Cu-BTC octahedra, followed by thermal decomposition, Cu/NC@Co/NC composites could be obtained.54 To further enhance the specific surface area of 3D MOF-derived carbons, the construction of hollow polyhedron structures is an important derivatization approach. Tannic acid and phytic acid are two common organic acid modifiers that can directly etch the internal entities of MOFs. Through chemical etching with suitable concentration and duration, MOFs would gradually form internal voids, with partial or complete destruction of crystal structure. Further carbonization can retain the original morphology, resulting in 3D hollow carbon structures (Figure 5D).51
Furthermore, many complex 3D MOF structures are formed through the stacking or self-assembly of nanorods, nanoplates, and other building blocks. Our group prepared a zeolitic tetrazolate framework (ZTF) with a nanoplate morphology formed by two organic ligands and Zn ions.55 Thereout, complex 3D micro-sized spheres can be obtained by self-assembly of these ZTF nanoplates (Figure 5E). This structure can be well retained during the carbonization process. Similarly, through a controlled hydrothermal reaction, Xu′s group transformed Zn-MOF-74 nanoparticles into hollow spherical 3D superstructures self-assembled from 1D nanorods.56
For 3D MOFs, there is a greater variety of ligand choices. And particular modifiers can further customize more complex nanostructures. Compared with 1D and 2D MOF-derived carbons, 3D MOF-derived carbon materials may face problems such as insufficient utilization of active metal sites buried in the interior and poor mass transfer. Chemical etching to form internal porous and even hollow structures will effectively solve these problems and provide more favorable platforms for further applications. These derived 3D carbon nanomaterials have been widely used in the field of catalysis, benefiting from advantages such as large specific surface area, uniform distribution of catalytic active sites, and excellent structural and chemical stability.
2.2 Component/Construction Modulation
Except for the morphology control, different heteroatom sources and structural modifiers can also be introduced into MOFs through the adjustment of ligands and modifiers. This allows for directed heteroatom doping and defect engineering of MOF frameworks during the thermal decomposition process.
2.2.1 Heteroatom Doping
First, organic ligands can be thermally decomposed into different heteroatom-doped/modified carbon materials. Ligands composed only of C, H, and O elements, such as p-phthalic acid57 and trimesic acid,58 typically give rise to O-modified carbon frameworks. While ligands containing N, S, and other heteroatoms, such as 2-methylimidazole59 and 1,4-dicarboxylbenzene-2,3-dithiol,60 can lead to other heteroatom-doped carbon materials. Owing to the electronegativity difference between these heteroatoms and carbon, the near-electroneutral carbon framework is effectively disrupted, resulting in enhanced overall conductivity and localized charge density. This would strengthen the interaction between the substrates and metallic active species, optimize the electronic structure of the active center, and enable the regulation of adsorption/desorption behaviors of electrocatalytic intermediates. Therefore, many studies choose to replace ligands composed of C, H, and O with ligands carrying other functional groups. Li′s group replaced BDC with BDC-NH2, resulting in derived single-atom Zn sites with different coordination structures (ZnO3C and ZnN4) in the carbon-based network.61 Cheng et al., chose to use two structurally similar ligands (1,4-dicarboxybenzene and 1,4-benzenedimethanethiol), obtaining partially substituted S-NiBDC structures (Figure 6A, B).62 The Ni2-S1 motifs in this MOF were similar to the Fe−S structure in [FeFe]-hydrogenase (Figure 6C). The introduction of S led to a significant positive shift in the p-band center, providing more states for bonding and antibonding orbitals (Figure 6D). For sure, these sulfhydryl groups can serve as sulfur dopants for the carbon-based network in the subsequent carbonization process.
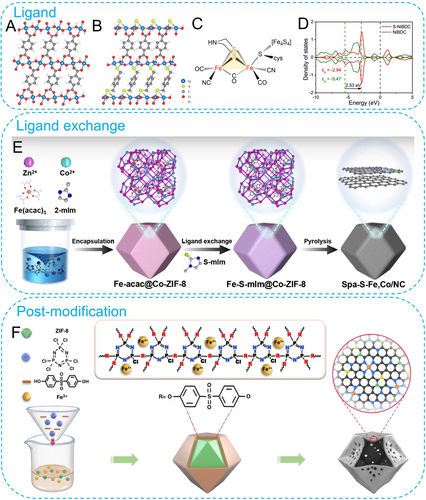
Structural configuration of (A) NiBDC and (B) S-NiBDC; (C) active site structure for [FeFe]-hydrogenase; (D) p-states partial density of states (PDOS) of these two models. Reproduced with permission.62 Copyright 2022, Nature Publishing Group. (E) Schematic of preparing Spa-S-Fe,Co/NC. Reproduced with permission.63 Copyright 2023, AAAS. (F) Illustration of preparation of Fe-SAs/NPS-HC. Reproduced with permission.67 Copyright 2018, Nature Publishing Group.
In addition to the introduction of heteroatom-containing organic ligands during the synthesis process, post-treatment ligand exchange over the MOF matrixes can also achieve diverse heteroatom doping. As shown in Figure 6E, introducing 1-methylimidazolidine-2-thione (S-mIm) into Fe-acac@Co-ZIF-8 framework through ligand exchange yields Fe-S-mIm@Co-ZIF-8.63 Further pyrolysis can result in spatial S-bridge ligand modified Fe−Co−N bimetallic centers. Moreover, ligand exchange strategies can also bring about some unique effects. Wu et al. employed pillared 4,4′-bipyridine (Bpy) for ligand exchange in the ZIF-67 framework, successfully inducing abundant linker defects.64 Besides the stable chelated Co2+ sites, the linker defects also bring about a large number of dangled Co2+ sites. The two different coordination environments lead to the formation of Co@CoO Mott–Schottky heterojunctions during the subsequent carbonization process.
Besides ligand modulation, the use of modifiers can directly introduce heteroatom dopants as well. Shang et al. mixed Cu-ZIF-8 with sulfur powder in a solvent, allowing sulfur to adsorb onto the surface of MOFs.65 During the carbonization process, S was doped into the carbon-based network and directly participated in the first-shell coordination of single-atom Cu. Similarly, pyridine-triphenylborane was incorporated as a B source into the Fe-ZIF-8.66 However, B only exists in the second-shell coordination configuration to modify central Fe−N4 sites in this work. Except for these small molecule modifiers, surface polymer modifiers can also effectively introduce heteroatoms. Li′s group performed polymer encapsulation on the surface of ZIF-8 using poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) and iron precursors (Figure 6F).67 The resulting hollow carbon-based network derived from the ZIF-8/Fe@PZS core–shell composites possessed abundant N, P, and S dopants, effectively modulating the microenvironment of single-atom Fe coordination.
2.2.2 Defect Engineering
Defect engineering of carbon-based frameworks primarily encompasses pore structures, hollow structures, and intrinsic carbon defects. In the research on MOF-derived carbons, the focus is mainly on the former two. Furthermore, direct ligand modulation is challenging to control the extra pore generation and hollow structure formation of carbon products. Therefore, the utilization of various structural modifiers is predominantly employed to achieve the desired effects.
For pore structures, the construction of macropores primarily relies on suitable templates. Shen et al. utilized polystyrene (PS) spheres as templates to directly construct ordered macro-microporous ZIF-8.68 The removal of PS templates resulted in the formation of macropores. Meanwhile, mesopores and micropores can be constructed using specific pore-forming agents. NH4Cl is an ideal pore-forming agent as it releases ammonia and hydrogen chloride gases during the pyrolysis process. Ammonia exhibits strong chemical etching properties towards carbon-based materials, effectively constructing a porous structure. Our group confined NH4Cl within the pores of ZIF-8 nanocubes (Figure 7A).69 Through thermal decomposition and acid washing, N-doped carbon nanocubes with well-developed porosity could be obtained. Such a porous carbon material could provide a suitable platform for the highly dispersed single-atom Ni sites. K+ ion also serves as an important pore-forming agent. Jiang's group employed K+ ion exchange to replace Me2NH2+ in bio-MOF-1.70 During the carbonization process, K+ ions could freely shuttle through the derived carbon-based network. The resulting K2O reacted with carbon atoms to generate gaseous products, leading to the formation of numerous microporous defect structures within the carbon matrix. The action mechanism of K+ has also been demonstrated in another study.71

(A) Preparation route of Ni-NC(p). Reproduced with permission.69 Copyright 2022, Wiley-VCH. (B) Fabrication process of HMCNCs and SMCNCs. Reproduced with permission.74 Copyright 2018, Wiley-VCH. (C) Formation of hollow structures through the “stress-induced orientation contraction” mechanism. Reproduced with permission.76 Copyright 2018, Wiley-VCH. (D) Glucose-assisted strategy for constructing the hollow carbonaceous materials. Reproduced with permission.78 Copyright 2018, American Chemical Society.
Hollow structures are the important targets for structural modification of MOF-derived carbons. Typically, a hollow structure effectively shortens the transfer path for substances and charges. Its large specific surface area provides more convenient pathways for mass transfer. Furthermore, the existence of hollow cavities allows active metal species to be exposed to the surface/subsurface that contact with electrolytes, affording more accessible active sites.72 As mentioned before, the strategy of using organic acids to etch MOF solids and obtain cavities has been introduced.73 Except for these organic acids, many structural modifiers can also play a role in the directed construction of hollow structures. As shown in Figure 7B, mesoporous silica (mSiO2) layers are coated on the surface of ZIF-8 nanocubes.74 A thin mSiO2 layer is unable to resist the inward contraction during carbonization and shrinks inward together with the derived carbon, resulting in the formation of porous carbon nanomaterials. In contrast, a thick mSiO2 layer possesses sufficient rigidity to effectively resist the tendency for inward contraction, leading to the outward contraction growth of derived carbon and ultimately obtaining a hollow structure. Similarly, coating mSiO2 layer on the surface of ZIF-67 polyhedron can also acquire hollow carbon nanostructures.75 Moreover, due to the induced growth by Co particles, massive carbon nanotubes are also grown on the surface of hollow carbon nanostructure, eventually forming a unique carbon composite framework.
Besides the inorganic structural modifiers like mSiO2, some organic modifiers also exhibit similar effects. After coating ZIF-8 surface with polydopamine (PDA), stress-induced orientation contraction can be achieved through the difference in decomposition temperatures between PDA and ZIF (Figure 7C).76 During the low-temperature range, the rapid decomposition of PDA forms a rigid surface layer, which serves a similar role as the mSiO2 coating layer. This would induce outward contraction of MOF-derived carbon during the following high-temperature treatment, resulting in the formation of hollow structures. Oligo-cyclotriphosphazene-co-hexahydroxytriphenylene (OCHT) oligomer shell and 4-aminophenol-formaldehyde (AF) polymer layers have also been reported to exhibit similar effects.77 Furthermore, glucose has been demonstrated to form carbonaceous composites under hydrothermal conditions. Wang et al. co-hydrothermalized glucose with ZIF-8, forming a carbonaceous composite layer on the MOF surface (Figure 7D).78 Subsequently, the released protons decomposed the internal ZIF-8 to obtain hollow metal-containing carbonaceous materials. Further carbonization could lead to highly graphitized hollow carbon nanomaterials.
The selection of ligands and modifiers can bring about different heteroatom doping to the derived carbons and significantly improve their chemical properties. However, for single-atom catalysts, the positions and configurations of these heteroatoms in the coordination shells are often very different. Many studies only infer from the characterization results, without giving explanations from the perspective of synthetic design. This problem still needs to be solved. Besides, various modifiers could bring more defect structures to MOF-derived carbons, effectively enhancing the proton, electron, and molecule transfer in electrochemical reactions. The development of such methods will provide more possibilities for the application of MOFs.
3 Metal Screening and Controlled Loading
By customizing MOF-derived carbons with appropriate morphologies, heteroatom doping, and defect structures, metallic active species can be uniformly dispersed and efficiently utilized. There are several sources of active metal species: 1) metal ions in the secondary building units (SBUs); 2) exogenous metallic modifiers introduced into the MOFs; 3) metal species incorporated into the as-derived carbons. In this section, we will summarize these three strategies for controlled metal loading to better understand their underlying principles.
3.1 Metal Nodes in MOFs
The most direct approach to introduce a specified metal element into the MOFs is to use it as a constituent element in the SBUs. If we desire to obtain the simple substances and compounds of a metal (M) over the MOF-derived carbons, the simplest method is to prepare M-MOF and then subject it to controlled thermal treatment under certain conditions. It is interesting to note the construction of isostructural MOFs, such as MIL-88 Fe/Ni, ZIF-8/67, MOF-5 Zn/Ni, MOF-74 Ni/Co. For example, ZIF-8 and ZIF-67 possess similar SBUs and the same organic ligands, thus exhibiting similar crystal structures. Therefore, during the synthesis process, the simultaneous introduction of Zn and Co ions will lead to the formation of ZnCo-ZIFs with the similar structure and morphology (Figure 8A–D).79 The Co nodes in ZnCo-ZIFs are well-spaced by Zn nodes, and strictly isolated during the thermal decomposition process, therefore obtaining a single-atom cobalt catalyst. Similarly, Fe in NH2−BDC−Fe can be partially replaced by Co, forming a bimetallic MOF composed of three different SBUs (trinuclear Fe3(μ3-O) cluster, Co3(μ3-O) cluster, and coordination unit between Co and −NH2) (Figure 8E).80 Heteroid metal atoms can be also synchronously introduced during the synthesis process. Lin et al. added Mn and Fe salts to the synthesis system of ZIF-8, in order to obtain Zn/Mn/Fe-ZIF (Figure 8F).81 The subsequent carbonization can result in Mn−Fe binary carbide (MnxFe3–xC) nanoparticles anchored by the NC matrix. However, it should be noted that the coordination of Mn and Fe with 2-methylimidazole does not result in isostructural MOF structures. Therefore, in this ternary MOF, whether Mn and Fe participate in the coordination by means of atomic substitution or are just adsorbed in the pores or on the surface of ZIF-8 needs more careful consideration.
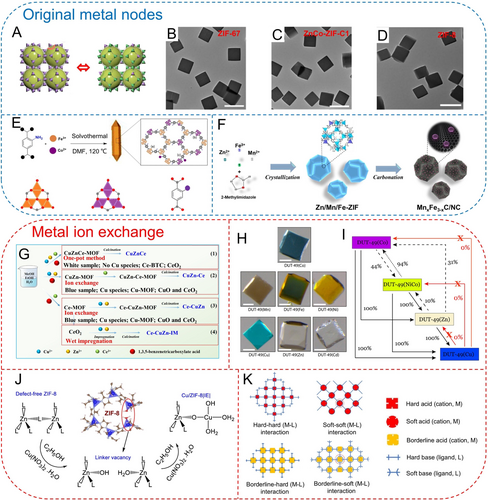
(A) Structural configuration of ZIF-67 and ZnCo-ZIF; TEM images of (B) ZIF-67, (C) ZnCo-ZIF-C1 and (D) ZIF-8. Reproduced with permission.79 Copyright 2023, American Chemical Society. (E) Synthetic schematic of Fe/Co-NH2-BDC with different secondary building units. Reproduced with permission.80 Copyright 2019, Wiley-VCH. (F) Synthetic schematic of Zn/Mn/Fe-ZIF and MnxFe3–xC/NC. Reproduced with permission.81 Copyright 2019, American Chemical Society. (G) Synthesis of CuZnCe series catalysts by different preparation routes. Reproduced with permission.82 Copyright 2024, Nature Publishing Group. (H) Optical images and (I) efficiency and competitive kinetics of pristine DUT-49(Co) and post-synthetic DUT-49(M). Reproduced with permission.83 Copyright 2020, American Chemical Society. (J) Schematic of Cu ion exchange process into the ZIF-8 framework. Reproduced with permission.84 Copyright 2024, Nature Publishing Group. (K) HSAB principle for cation exchange in MOFs. Reproduced with permission.85 Copyright 2020, Elsevier.
As mentioned above, some metal ions can hardly form the desired MOF structure with specified ligands. However, metal ion exchange strategies provide an effective solution that can greatly enrich the variety and properties of MOFs. By cation exchange, multiple metal species can be incorporated into MOFs, thereby obtaining complex multi-metal active species. Ye et al. discovered that coordinating Cu, Zn, and Ce salts with trimesic acid by a one-pot method only resulted in a white sample without any Cu species (Figure 8G).82 XRD analysis revealed that the crystal structure was Ce-BTC, and the thermal decomposition product was only CeO2. However, conducting Ce or Cu/Zn ion exchange on the pre-obtained CuZn-MOF or Ce-MOF could obtain blue samples containing Cu species. The crystal structure exhibited the Cu-MOF structure, and the thermal decomposition products were CuO and CeO2. This indicates that the ion exchange strategy is sometimes indispensable for obtaining specific MOF structures and derivatives. Garai et al. demonstrated the universality of metal ion exchange strategy on the DUT-49 framework.83 Based on DUT-49 (Co), various metals such as Mn, Fe, Ni, Cu, Zn, and Cd can selectively replace the Co sites without altering the crystal structure of the MOF (Figure 8H). Furthermore, the authors also found that such metal ion exchange processes often had an upper limit on the exchange degree, and some conversion pathways were not feasible (Figure 8I).
Notably, the ion exchange process is fundamentally different from direct wet impregnation. Velisoju integrated Cu species into ZIF-8 framework by both ion exchange and wet impregnation strategies (Figure 8J).84 In the ion exchange process, structural defects could be formed with partial linker release in the MOF structure. The missing linkers were then replaced by hydroxyl groups, while Cu was anchored to these hydroxyl ligands. These missing linkers and metal defects can be detected through infrared spectroscopy. Differently, the wet impregnation could only confine the Cu ion within the porosity or on the surface of ZIF-8 without any stable coordination. Such a process may also result in a loss of accessible porosity. Therefore, characterization techniques sensitive to functional groups and coordination information, are crucial for distinguishing these two different processes.
There is currently no consensus on the fundamental principles of cation exchange in MOFs. A possible and widely used hypothesis is the hard-soft acid-base (HSAB) principle.85 According to this theory, metal cations are generally considered as Lewis acids (hard acids: high-valence cations such as Zr4+, Cr3+, Fe3+; borderline acids: Cu2+, Fe2+, Co2+, Ni2+, Zn2+; soft acids: Ag+ and Au+). While organic ligands could be considered as Lewis bases with different hardness (Figure 8K). MOF structures are Lewis acid-base adducts, where different metals and ligands have different hardness and softness. In general, soft bases tend to bind well with soft acids, while hard bases exhibit a good affinity for hard acids. The coordination bond strength or stability of MOFs is to some extent dependent on the softness or hardness of Lewis acids and bases. Understanding the differences in softness/hardness enables the prediction or guidance of cation exchange in MOFs. For example, in stable hard-hard PCN-333(Fe), Cr ion exchange can occur because Cr3+ is harder than Fe3+.86 Furthermore, in some unstable MOFs involving unstable borderline acid-hard base coordination (MOF-5, HKUST-1), the hard-acid cations exchange process is more favorable to occur.87 It is worth noting that metal ion exchange often requires the assistance of solvents, temperature, and pressure to facilitate the reaction.88
3.2 Confinement Effect of Pore Structure in MOFs
Pristine MOFs possess well-developed pore structures that can accommodate various metal-containing modifiers, thereby obtaining active metal species in derived carbons. Depending on the pore size, different modifiers ranging from metal ions to relatively large molecules can be accommodated. Zhao et al. confined Ni precursors within the pores of ZIF-8, where the hexagonal window size was approximately 3.3 Å (Figure 9A).89 During the carbonization process, Zn species gradually evaporated, leaving behind defect sites. While the confined Ni species gradually coordinated with N atoms in the carbon framework, ultimately forming single-atom Ni configurations. Such a simple wet impregnation-pore confinement-carbonization strategy has been widely developed for obtaining diverse metal-heteroatom-carbon nanomaterials.90
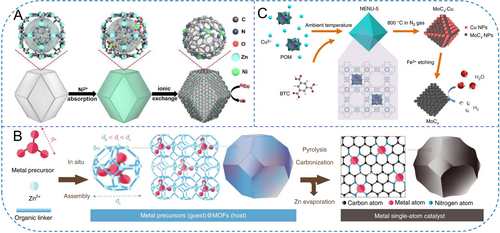
(A) Scheme of the synthesis of Ni SAs/N-C. Reproduced with permission.89 Copyright 2017, American Chemical Society. (B) Host-guest strategy for synthesizing single-atom Ir1/CN. Reproduced with permission.91 Copyright 2020, Nature Publishing Group. (C) Synthesis of NENU-5 with Mo-based POMs residing in the pores of HKUST-1 framework and post-treatments. Reproduced with permission.92 Copyright 2015, Nature Publishing Group.
Besides metal ions, appropriate guest molecules can be also trapped within the pores. Li′s group introduced appropriately sized Ir(acac)3 during the synthesis of ZIF-8 (Figure 9B).91 The guest molecule's size is larger than the pores of ZIF-8 but smaller than the overall cavities, allowing it to be securely confined within the pore and serve as precursors for single-atom Ir on NC substrates. Besides, NENU-5 is a unique MOF structure based on the architecture of HKUST-1 (Cu3(BTC)2(H2O)3). Mo-based Keggin-type POMs (H3PMo12O40), as the guest molecules, were periodically confined within its largest pores, resulting in a novel [Cu2(BTC)4/3(H2O)2]6[H3PMo12O40] structure (Figure 9C).92 This novel MOF structure can be derivatized into Mo−Cu bimetallic-doped carbon-based catalysts.
The confinement effect of pore structure is an important and controllable method for metal loading, which has been extensively studied.93 Fine-tuning the size of pore structures and selecting appropriate metal-containing guest modifiers can significantly enrich the variety, morphology, and loading capacity of metals in MOF derivatives.
3.3 Directed Assembly over the MOF-Derived Carbon Matrixes
Apart from the metal modification within the pristine MOF precursors, controlled metal loading can be also achieved by further assembling active metal species directly on their thermally derived carbon products.
Due to the presence of metal species within many MOF derivatives, in situ metal exchange strategies can be employed to introduce additional metal species or achieve complete substitution. Li et al. first carbonized Cu(btc)3 under an inert atmosphere to obtain Cu@porous carbon material (Figure 10A).94 Subsequently, in a solvothermal reaction with filled nitrogen, Cu nanoparticles were gradually replaced by Sb element (3Cu+SbCl3→3CuCl+Sb), resulting in the in situ formation of ultrafine Sb nanoparticles supported on porous carbon materials. As for metal-free carbon frameworks derived from MOFs, the adsorption-annealing strategy can be utilized to introduce additional active metal components owing to the inherited porous structure. Zhang's group employed a wet chemical method to adsorb Fe(phen)32+ complexes onto carbon matrixes derived from NH2-MIL-101(Al) and MOF-5, respectively (Figure 10B).95 Further annealing could result in single-atom Fe sites supported on porous carbon-based nanomaterials. Yuan and co-workers adsorbed various precious metal species, such as Ru3+, Pt4+, and Pd2+, onto Co-SAs/NC materials derived from bimetallic ZnCo-ZIF in an aqueous solution.96 After low-temperature annealing, a single-atom Co−N−C nanocatalyst with ultra-small Ru/Pt/Pd nanoclusters could be obtained. In addition to the adsorption effect in wet chemical methods, the adsorption-annealing process may also occur in other systems. Chen et al. integrated the adsorption and annealing processes into a one-step operation by introducing SiH4 gas as the silicon source during the secondary pyrolysis of carbon nanomaterials derived from ZIF-8.97 Owing to the confinement effect, controlled growth of Si nanodots was induced within the channels.
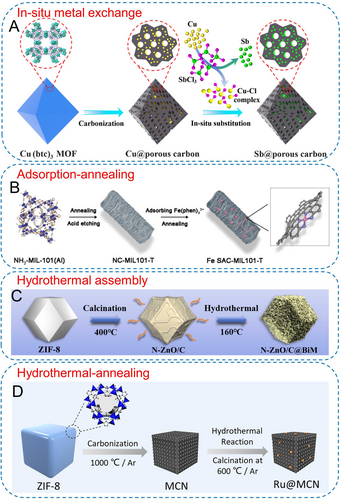
(A) Scheme of the synthetic route of Sb@PC. Reproduced with permission.94 Copyright 2021, American Chemical Society. (B) Schematic of the synthesis of Fe SAC-MIL101-T. Reproduced with permission.95a Copyright 2021, Wiley-VCH. (C) Hydrothermal self-assembly for synthesizing N-ZnO/C@BiM. Reproduced with permission.98 Copyright 2022, Elsevier. (D) Synthetic scheme of Ru@MCN. Reproduced with permission.100 Copyright 2021, American Chemical Society.
Utilizing MOF-derived carbons as substrates, directed assembly of metal components on their surfaces through hydrothermal/solvothermal processes is a common post-modification approach. Wang et al. thermally decomposed ZIF-8 at a low temperature to obtain a N-doped ZnO carbon skeleton.98 Subsequently, hierarchical Bi2MoO6 (BiM) nanosheets were directly grown on its surface through a hydrothermal process, forming the S-scheme heterojunction (Figure 10C). Furthermore, with ethylene glycol (EG) as the reducing agent, RuIr alloys and single-atom Pt sites were rapidly reduced onto MOF-derived cobalt-nitrogen-carbon and hollow porous carbon nanorods at 180 and 200 °C, respectively.99 Importantly, the products obtained through hydrothermal/solvothermal processes are sometimes amorphous and require secondary heat treatment for crystallization. Wei et al. loaded Ru species onto MOF-derived microporous carbon nanocubes through a hydrothermal process, followed by pyrolysis at 600 °C, resulting in Ru nanoparticles with a loading of ~10 wt % (Figure 10D).100
It is evident that the directed assembly of various active metal species into MOF-derived carbons is the simplest means of introducing active materials. This approach will significantly enrich the variety of MOF derivatives and provide avenues for constructing hybrid, composite, and heterostructure catalytic active centers.
4 Recent Progress in Electrochemical Reduction Reactions
MOF-derived carbon-supported metal catalysts can be employed in various electrocatalytic reactions owing to some unique advantages: 1) tunable metals and ligands that can endow derivatives with different morphologies and compositions; 2) the inherited well-developed pore structure that assures the mass transfer; 3) high conductivity that facilitates the electron transfer process; 4) adequate active metal components serving as necessary catalytic centers; 5) strong metal-support interaction further regulating the electronic structure of active species and providing armor-like protection.
As mentioned above, MOF-derived carbon-supported metal electrocatalysts are more suitable for electrochemical reduction reactions. This is primarily attributed to the outstanding stability of both active metal species and carbon substrates under reduction potentials, enabling the constantness of active centers and structural morphology. Conversely, under oxidation potentials, metal species are susceptible to oxidation, gradually forming oxygenated compounds.101 While the carbon substrates are prone to oxidation and subsequent corrosion.17a, 102 The inherent variability of catalytic centers and the instability of substrates often lead to a gradual decline in catalytic activity, making it challenging to achieve long-term, high-current industrial applications. Therefore, this section aims to provide a comprehensive summary of the recent applications of MOF-derived carbon-supported metal electrocatalysts in energy-related electrochemical reduction reactions.
4.1 Hydrogen Evolution Reaction (HER)
Hydrogen generation from electrocatalytic HER is an important part in artificial hydrogen cycle. HER involves the dissociation of water molecules into hydrogen product. In acidic media, due to the abundance of protons, the formed hydronium cations directly adsorb onto the active sites of the electrode, forming M-H* intermediates. In alkaline media, since protons originate from water molecules, an additional water dissociation step is required to form M-H* within the electrode's double layer. These steps are the electrochemical hydrogen adsorption process, known as the Volmer reaction.103 Subsequently, there are two reaction mechanisms. One is the electrochemical desorption process, where the M-H* intermediates react with another proton (acid) and water molecule (base) to form H2 molecules. The other is the chemical desorption process, where adjacent M-H* species directly combine to form H2 molecules.104 In terms of HER catalysis, active metal species are almost essential components, exhibiting higher activity compared to non-metal catalysts. MOF-derived carbon-supported metal catalysts can host different active metal centers, and their well-developed pore structures provide effective pathways for mass and charge transport. Therefore, they can be widely developed for HER electrocatalysis.
Our group conducted galvanic replacement of Pt ions on Co@NCNT derived from ZIF-67@PDA nanorods, resulting in the formation of two different types of Pt3Co and PtCo nanoparticles loaded on nitrogen-doped carbon nanotubes (Pt3Co@NCNT and PtCo@NCNT).29 The atomic-scale scanning transmission electron microscopy (STEM) analysis revealed the regular arrangement of Pt and Co atoms in these two different structures (Figure 11A, B). In acidic and alkaline media, Pt3Co@NCNT exhibited superior HER catalytic activity compared to PtCo@NCNT and commercial Pt/C catalysts, achieving benchmark current densities of 10 mA cm−2 with overpotentials as low as 42 and 36 mV, respectively (Figure 11C, D). Density functional theory (DFT) calculations showed that the alloying effect between Pt and Co atoms significantly optimized the adsorption strength of hydrogen intermediates on the active sites. On the Pt3Co (111) facet, the alloy composition and synergistic effect induced diverse catalytic active sites. In another study, the thermal decomposition of Pt-ZIF-67 resulted in the CoPt alloy and isolated Pt-N2C2 sites, and the strong interaction between them caused a positive shift of the d-band center.105 Thus, the binding strength of H* was enhanced and the adsorption barrier was then reduced. Pan et al. synthesized carbon-encapsulated CoNiPt alloy catalysts by thermolysis of Hofmann-type MOFs with different morphologies and Pt contents (Figure 11E).106 Significant compositional segregation occurred in this catalyst, forming CoNi-rich and Pt-rich phases. The catalyst with a Pt loading of 15 wt % exhibited excellent alkaline HER catalytic activity with an overpotential of only 25 mV at 10 mA cm−2 (Figure 11F). Corresponding DFT simulations demonstrated that the CoNi-rich phase accelerated the dissociation of water molecules in alkaline media, followed by hydrogen spillover to the Pt-rich phase where the adsorption energy of H* intermediate was optimized (Figure 11G, H).
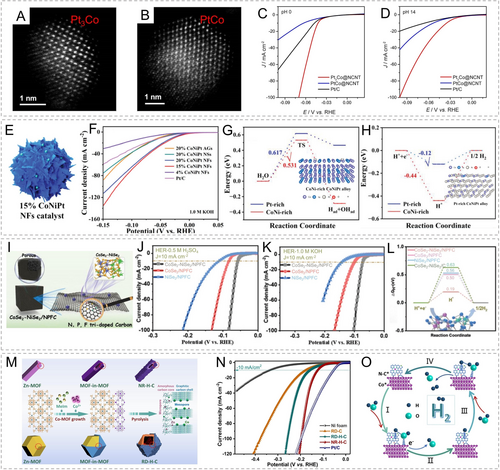
STEM images of (A) Pt3Co and (B) PtCo nanoparticles; HER polarization curves of Pt3Co@NCNT, PtCo@NCNT and commercial Pt/C in (C) acidic and (D) alkaline media. Reproduced with permission.29 Copyright 2021, Wiley-VCH. (E) Schematic of 15 % CoNiPt NFs catalyst; (F) alkaline HER activity of different catalysts; (G) water dissociation energy barrier and (H) hydrogen adsorption free energy of CoNi-rich and Pt-rich phase alloy. Reproduced with permission.106a Copyright 2023, Wiley-VCH. (I) Schematic of CoSe2-NiSe2/NPFC; HER performance of CoSe2-NiSe2/NPFC, CoSe2/NPFC, and NiSe2/NPFC in (J) acidic and (K) alkaline media; (L) ▵GH* free energy diagram of different catalysts. Reproduced with permission.110 Copyright 2023, Wiley-VCH. (M) Scheme of the synthesis of NR-H-C and RD-H-C; (N) Polarization curves of different catalysts; (O) scheme of the Volmer–Heyrovsky mechanism on NR-H-C in alkaline media. Reproduced with permission.112 Copyright 2022, Elsevier.
Besides Pt, other noble metal elements also exhibit notable HER catalytic activity.107 Cai et al. constructed active alloy phases of various Pt-group metals with Co in a carbon-based framework derived from truncated rhombic dodecahedrons ZIF-67. The activity trend observed was PtCo/C>PdCo/C>RuCo/C. Moreover, Zhou et al. prepared an IrCo alloy phase on a 2D MOF-derived carbon substrate (IrCo@NC-850).108 In alkaline and acidic media, it achieved the current density of 10 mA cm−2 with overpotentials of merely 82 and 50 mV, respectively.
Apart from these catalysts containing active noble-metal components, non-precious metal-based HER catalysts have also been extensively explored.109 Zhang's group acquired different N, P, and F triple-doped carbon nanostructures with CoSe2-NiSe2 heterojunctions through the modulation effect of ionic liquids (Figure 11I).110 The CoSe2-NiSe2/NPFC catalyst with cubic morphology exhibited well-developed pore structures and distinct CoSe2-NiSe2 heterointerfaces. The benchmark overpotentials in acidic and alkaline media were 57 and 86 mV, respectively, outperforming the comparative samples with single metal selenide components (Figure 11J, K). The enhancement in HER catalytic activity was mainly attributed to the significantly optimized d-band structure and induced charge density redistribution at the heterointerfaces. This led to the optimal Gibbs free energy for H* and improved adsorption strength for OH*, thereby reducing the reaction barriers (Figure 11L). Such unique heterostructures between different metal components have become a recent research focus, providing insights into the synergistic effects among multiple components and the underlying reasons for enhanced catalytic activity.
Certainly, single-phase non-noble-metal active components or doped monophasic components can also exhibit decent HER catalytic activity.111 Dang et al. achieved controlled modulation of MOF precursors to prepare two different morphologies of MOF-in-MOF structures (Figure 11M).112 The structures of the derived materials were hollow nanorods (NR-H-C) and rhombic dodecahedrons (RD-H-C), with both containing Co nanoparticles as the active metal component. In alkaline media, NR-H-C demonstrated superior HER catalytic activity (η10=123 mV) to RD-H-C (η10=159 mV) (Figure 11N). This indicates that the diverse structural morphologies derived from MOFs lead to disparate mass and electron transfer processes, as well as influence the exposure and utilization of catalytic active sites (Figure 11O).
As for the application of MOF-derived carbon-supported metal-based catalysts in HER, the current research direction is mainly aimed at reducing the amount of platinum-group active metal substances or developing non-precious metal-based alternatives. Precious metal-based catalysts still possess advantages in overpotential and turnover frequency, but the development of non-precious metal catalysts seems more meaningful in the long run. Compared with traditional overpotentials, indexes such as mass and price activities are more suitable for large-scale commercial applications. For the HER catalytic mechanism, moderate hydrogen adsorption strength and low energy barrier of water dissociation are the ultimate goals. The design of catalytic centers and the relevant implementation of synthesis are important research ideas that are relatively missing in many literatures.
4.2 Oxygen Reduction Reaction (ORR)
ORR is the rate-limiting step in many electrochemical apparatuses. Depending on the number of electrons involved, it can be classified as a four-electron or a two-electron process. The four-electron process converts oxygen into water molecules, while the two-electron process converts oxygen into hydrogen peroxide molecules.113 Therefore, besides the catalytic activity, selectivity is also an important evaluation criterion for ORR catalysts. Furthermore, the variety of acidic or alkaline media would greatly affect the catalytic activity and selectivity. This part will summarize the applications of MOF-derived carbon-supported metal electrocatalysts in ORR in four different scenarios.
Hydrogen peroxide (H2O2) is an important chemical feedstock, and its electrochemical production has attracted significant attention due to its green and environmentally friendly nature.114 In acidic media, H2O2 exhibits strong oxidation capability and high stability. It is also compatible with well-established proton exchange membrane devices, making the electrocatalytic synthesis of H2O2 in acidic ORR highly feasible.115 The two-electron ORR catalytic pathway in acidic media is as follows: O2+*+(H++e−)→*OOH; *OOH+(H++e−)→H2O2+*. In a previous study, researchers conducted a comprehensive analysis of two-electron ORR activity and selectivity of different transition metal-nitrogen-carbon catalysts in acidic media, by combining theoretical and experimental results. All the synthesized carbon materials were derived from ZIF-8. The results showed that the Co−N−C catalyst was the most promising candidate for acidic two-electron ORR catalysis, with the activity trend as Co−N−C>Ni−N−C>N−C>Mn−N−C>Cu−N−C>Fe−N−C.116 Therefore, Co-based metal species have been widely developed for acidic two-electron ORR catalysis.117 Huang et al. first predicted the theoretical catalytic activity and selectivity of different single-atom CoN4–xOx and diatomic Co2N6–xOx sites for two-electron ORR through theoretical calculations.118 The results indicated that Co2N4O2 exhibited the most appropriate *OOH adsorption strength and thus the lowest reaction barrier for two-electron ORR, as compared to other diatomic Co-based configurations and other O-bridge dual metal centers (such as Fe2−, Ni2−, Cu2−, Pd2−N4O2) (Figure 12A). In practical rotating ring-disk electrode (RRDE) tests, the as-prepared Co2-DAC catalyst with the Co2N4O2 motif exhibited extremely high H2O2 selectivity (>95 %) in 0.1 M HClO4 solution (Figure 12B, C). The average number of consumed electrons was 2.1, and it provided a partial current density for H2O2 of up to 2.7 mA cm−2 at the voltage range of 0.17 to 0.20 V versus reversible hydrogen electrode (vs. RHE). In flow-cell tests, the catalyst maintained an H2O2 Faradaic efficiency (FE) of over 78 % at both low and high current densities, with a peak H2O2 production rate of 11.72 mol gcat−1 h−1. Furthermore, the cumulative yield of H2O2 increased nearly linearly with time, indicating excellent stability (Figure 12D).
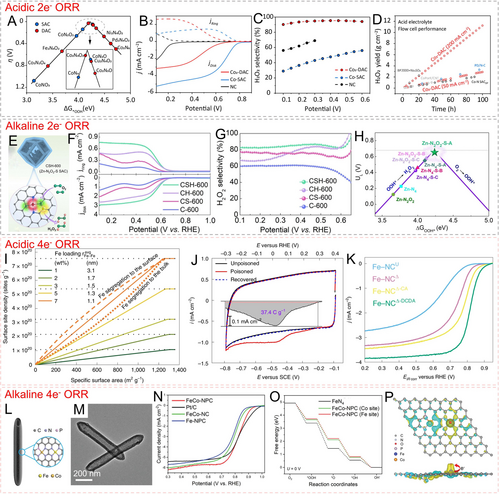
(A) 2e− ORR volcano plot for different single-atom catalysts (SACs) and double-atom catalysts (DACs); (B) RRDE polarization curves of Co2-DAC, Co-SAC, and N−C in acidic media; (C) H2O2 selectivity at different potentials of these catalysts; (D) accumulated H2O2 yield of Co2-DAC and some reported catalysts in a flow cell. Reproduced with permission.118 Copyright 2024, American Chemical Society. (E) Schematic of Zn−N2O2−S catalyst; (F) RRDE polarization curves and (G) H2O2 selectivity of the catalysts in alkaline media; (H) 2e− ORR volcano plot of different models. Reproduced with permission.119 Copyright 2023, Wiley-VCH. (I) Calculated surface site density as a function of specific surface area for SACs with different Fe loading; (J) CV curves of Fe-NCΔ−DCDA catalyst at un-poisoned, poisoned and recovered phases during nitrite stripping test; (K) ORR curves of different catalysts in acidic media. Reproduced with permission.126 Copyright 2022, Nature Publishing Group. (L) Schematic and (M) TEM image of FeCo-NPC; (N) LSV curves of different catalysts in alkaline media; (O) ORR free energy diagram of different models; (P) differential charge density of *O adsorbed on Fe site of FeCo-NPC model. Reproduced with permission.130 Copyright 2023, Wiley-VCH.
The development of two-electron ORR catalysts in alkaline media is currently a hot research topic. Many reports focus on modulating the selectivity of alkaline ORR based on Zn-based and Co-based active species. Wei et al. utilized organic acids containing catechol to etch the ZIF-8 framework, followed by low-temperature pyrolysis and sulfur doping, to obtain the CSH-600 catalyst with well-defined Zn−N2O2−S sites (Figure 12E).119 In alkaline media, this catalyst exhibited an enhanced disk current density close to the theoretical mass transport limit of two-electron ORR catalysis (Figure 12F). Furthermore, CSH-600 showed over 80 % H2O2 selectivity over a wide potential range, attributed to the moderate adsorption strength of OOH* on its catalytic active sites (Figure 12G, H). Catalytic active centers such as single-atom Co-O4,120 ZnO3C,61 and ZnN3O121 have also been reported to exhibit excellent alkaline two-electron ORR selectivity. In addition to non-noble metals, some noble metals have also been employed as alkaline two-electron ORR catalysts. Wang et al. constructed single-atom Pd−N4 sites on ZIF-derived carbon and demonstrated the efficient catalytic production of H2O2 in alkaline media.122 Pd−N4 is believed to favor the production of H2O2 (−O breaking) rather than H2O (O−O breaking) generation.
The four-electron ORR is a crucial reaction at the cathode of fuel cells. The most mature proton exchange membrane fuel cell (PEMFC) technology requires efficient and durable four-electron ORR catalysts. Precious Pt-based nanomaterials are the most commonly used acidic four-electron ORR catalysts.123 Gong et al. anchored ultralow Pt nanoclusters onto a carbon framework with single-atom Mn−N4 sites.124 The strong interaction between these two components significantly limits the overgrowth of Pt species and induces electron transfer from the Mn sites to the Pt species, resulting in a lower reaction barrier for the four-electron ORR. Due to the low Pt loading and high catalytic activity, this catalyst exhibits a high mass activity at 0.9 V vs. RHE in acidic media, which is 11.1 times that of commercial Pt/C catalysts. Certainly, non-precious metal-based catalysts are more in line with the requirements of sustainable development.95b, 125 Mehmood et al. first analyzed the evolution of site density (SD) for single-atom Fe under different iron loadings and specific surface areas (Figure 12I).126 In carbon-based materials with high loadings (7 wt %, namely 1.6 at%) and specific surface areas (~1000 m2 g−1), a site density of approximately 7.5×1020 sites g−1 can be achieved. This is highly instructive for guiding the site density engineering of single-atom catalysts. Accordingly, the authors acid-etched the thermal decomposition product of Zn-MOF, and anchored iron precursors within the remaining vacancy defects. With the assistance of dicyandiamide (DCDA), secondary thermal activation was performed to obtain a single-atom Fe catalyst with a loading of 7 wt %. In situ nitrite stripping tests were conducted on this catalyst to analyze its site density, which was approximately 4.67×1019 sites g−1 (4.04×1016 sites m−2), accounting for only 6 % of the theoretical value (Figure 12J). This catalyst exhibits a half-wave potential (E1/2) as high as 0.815 V vs. RHE and excellent four-electron selectivity in acidic media (Figure 12K). As a result, in H2−O2 fuel cells, this catalyst can reach a peak power density of >1.2 W cm−2 at a cell voltage of 0.49 V. The authors point out that if the single-atom Fe sites carried by the catalyst can be more efficiently utilized, the acidic ORR activity can be greatly enhanced.
For the four-electron ORR in alkaline media, non-precious metal-based catalysts have exhibited catalytic activity comparable to commercial Pt/C catalysts. Iron-based and cobalt-based nanomaterials are the most important catalyst candidates.127 Chang et al. mechanically mixed MIL-101(Fe) and ZIF-8 in certain proportions, followed by calcination with a phosphorus source, to obtain Fe/N/P-tridoped expanded carbon nanotubes with anchored Fe3C and Fe2P nanoparticles.128 In alkaline media, this catalyst exhibits a half-wave potential of 0.88 V vs. RHE and demonstrates excellent four-electron selectivity. Yuan et al. calcinated a carboxylate/amide mixed-ligand Zn-MOF (DMOF), followed by a gas transport method, to obtain vacancy-defect-modified CoN4 sites on a pumice-like carbon nanomaterial.129 Such a catalyst exhibits an E1/2 of 0.866 V vs. RHE and excellent zinc-air battery performance in alkaline media. Multi-metal active component strategies can further modulate the electronic structure and catalytic activity of integrated catalytic centers. Our group utilized phytic acid to etch FeCo NH2-MIL-88B, forming hollow nanotube morphology (Figure 12L, M).130 The N and P-regulated dual-atom catalytic centers induced polarization-distributed surface charges, and optimized the adsorption/desorption behavior of oxygen intermediates, ensuring excellent four-electron ORR catalytic activity in alkaline media (Figure 12N–P).
In these studies, the active metal species can be single-component or multi-component, and their configuration can be single atoms, nanoclusters, or nanoparticles.131 The analysis of different catalytic centers is of significant importance in guiding the development of catalysts tailored for specific application scenarios. For both two-electron and four-electron ORR catalysis, a large number of MOF-derived carbon-supported metal catalysts have been widely reported, but actually, very few catalysts can be industrially applied. This is mainly limited by the durability issues of the carbon framework and active metal substances under operating conditions. Attempting to rethink the design of MOF-derived carbon-supported metal catalysts based on the structural composition of commercial catalysts may provide insights for their future development.
4.3 Carbon Dioxide Reduction Reaction (CO2RR)
The carbon-neutral climate targets and the decreasing cost of renewable electricity have made electrochemical CO2RR a promising option for addressing the CO2 crisis.132 CO2RR provides a novel technological approach for the artificial carbon cycle, which not only reduces CO2 emissions but also offers certain fuels or high-value-added chemicals. The main products include gaseous fuels (carbon monoxide, methane, etc.), liquid C1 products (formic acid, methanol, etc.), and liquid Cn (n≥2) products (ethanol, acetone, etc.).133 However, electrochemical CO2RR faces significant challenges, including the high activation energy barrier of CO2 molecules, competition with the HER, and selectivity limitations imposed by multi-electron-proton (or hydroxyl) coupled transfer steps. In previous studies, MOF-derived carbon-supported metal electrocatalysts have been widely employed in CO2RR, leading to various reaction pathways and diverse product distributions.134
For gaseous products, CO has been extensively studied. Different metal-based catalysts, particularly those in the form of single-atom sites, have been widely reported to exhibit excellent activity and selectivity towards CO2RR.135 In an early study, a single-atom Fe3+-N-C catalyst was obtained by calcining Fe-doped ZIF-8.136 This catalyst showed CO production at a low overpotential of 80 mV and achieved a CO partial current density of 94 mA cm−2 at the overpotential of 340 mV. The catalytic sites could maintain a +3 oxidation state during the reaction, and possess faster CO2 adsorption and weaker CO adsorption, thus demonstrating superior CO2-to-CO activity. Our group carbonized a Zn-based dual-linker zeolitic tetrazolate framework, followed by Ni loading, to obtain single-Ni sites.55 The 5-aminotetrazole ligand could in situ construct a porous structure of nanoplate-assembled microspheres (Figure 13A). The resulting catalyst exhibited nearly 100 % CO selectivity over a wide voltage range and demonstrated excellent long-term stability (Figure 13B, C). Besides CO, CH4 is also an important gaseous product. Sun′s group obtained a Cu/CeO2@C catalyst by calcining CeCu-BTC under an inert atmosphere.137 This catalyst achieved a FE for CH4 production as high as 80.3 %, and a maximum CH4 partial current density of 138.6 mA cm−2. Through operando attenuated total reflection-infrared spectroscopic analysis, it was found that MOF-derived carbon facilitated charge transfer, enhanced the adsorption of intermediates on active sites, improved reaction kinetics, and ultimately enhanced the catalytic selectivity for CH4.
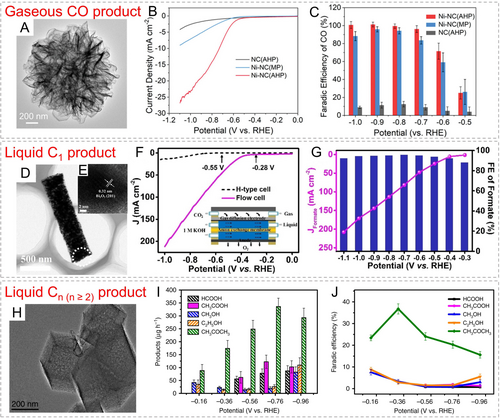
(A) TEM image of Ni-NC(AHP); (B) polarization curves of Ni-NC(AHP), Ni-NC(MP) and NC(AHP) in CO2-saturated 0.5 M KHCO3 electrolyte; (C) CO FE of these catalysts at different potentials. Reproduced with permission.55 Copyright 2021, Wiley-VCH. (D, E) TEM images of Bi2O3@C-800; (F) LSV curves in H-type and flow cells of Bi2O3@C-800; (G) FE and partial current density of formate at different potentials for Bi2O3@C-800. Reproduced with permission.138 Copyright 2020, Wiley-VCH. (H) TEM image of Cu-SA/NPC; (I) Production rate and (J) FE of different CO2 reduction products on Cu-SA/NPC catalyst. Reproduced with permission.142 Copyright 2020, Nature Publishing Group.
Liquid products can be divided into single-carbon and multi-carbon products. Among the single-carbon products, formic acid (HCOOH) or formate (HCOO−) is the important liquid product generated through a two-electron process, and the reaction mechanism may involve three pathways: 1) the carbon atom in CO2− combines with the catalytic site, and the oxygen atom undergoes protonation to form *COOH intermediate; 2) both oxygen atoms in CO2− bind to the catalytic site, and protonation occurs on the carbon atom to form HCOO* intermediate; 3) based on the pathway 2, protons and electrons from HCOO* gradually migrate to form *OCHO intermediate.133 Xia′s group reported the Bi2O3 nanoparticles encapsulated on carbon nanorods derived from Bi-BTC (Figure 13D, E).138 This catalyst effectively electrochemically converted CO2 into formate with a low onset potential of −0.28 V vs. RHE and a current density exceeding 200 mA cm−2 (Figure 13F). It also exhibited a high CO2-to-formate FE of 93 % in a flow cell (Figure 13G). The presence of Bi2O3 accelerated reaction kinetics and enhanced formate selectivity, while the carbon matrix contributed to increased activity and partial current density for formate production. Qiu et al. reported In2O3–x@C derived from MIL-68(In) as a catalyst for electrochemical reduction of CO2 to formic acid.139 The abundant oxygen vacancies in In2O3–x particles exposed more active In3+ sites, which favored the formation of HCOO* intermediates. As a result, this catalyst exhibited an 84 % FE at a low potential of −0.4 V vs. RHE, with a HCOOH partial current density of 11 mA cm−2. Furthermore, Payra et al. constructed PtxZn nanoalloys on ZIF-8-derived carbon matrixes.140 The mixed-phase PtxZn/C catalyst demonstrated excellent selectivity for electrochemical CO2 reduction to CH3OH. It facilitated the transfer of electrons to adsorbed CO2 molecules and better interact with the CO2⋅− intermediate. Moreover, the weak binding strength between PtxZn/C and −OCH3 greatly enhances the catalyst's selectivity towards CH3OH.
Liquid multi-carbon products refer to organic molecules containing two or more carbon atoms, and their production is predominantly based on Cu-based electrocatalysts. For different multi-carbon products, the intermediates, the number of electron-proton transfers involved, and the underlying reaction mechanisms vary. Zhao et al. obtained oxide-derived Cu/carbon (OD Cu/C) material through the carbonization of HKUST-1.141 This catalyst exhibited the ability to efficiently reduce CO2 to alcohol compounds within a wide voltage range of −0.1 to −0.7 V vs. RHE, with a total FE ranging from 45.2 % to 71.2 %. Specifically, the onset potential for the production of two-carbon ethanol was as low as −0.1 V vs. RHE, with a yield of 3.7 to 13.4 mg L−1 h−1. Furthermore, Zhao and co-workers synthesized Cu-SA/NPC catalyst with isolated metal sites from Cu-doped ZIF-8, and investigated its performance in CO2 reduction to multi-carbon products (Figure 13H).142 Acetone was the main reduction product, with a FE of 36.7 % and a yield of 336.1 μg h−1 (Figure 13I, J). The distinctive Cu-pyrrolic-N4 active sites contributed to stabilizing the critical intermediate for acetone formation, and the synergistic effect of Cu−N bonds promoted C−C coupling reactions.
The selectivity of electrochemical CO2RR fundamentally depends on the relative binding strength of key intermediates such as *COOH, *OCHO, *CO, and *H on the surface of catalysts. Therefore, MOF-derived carbon-supported metal materials, with their high tunability in terms of structure, metal composition, and coordination environment, offer abundant possibilities for obtaining highly selective CO2RR catalysts. However, for such catalysts, the active center may undergo dynamic evolution at high reduction potentials, leading to reversible/irreversible changes in configuration, composition, and phase of active species. In addition, the formed surface adsorbed layer during this process would also significantly affect the activity and selectivity of catalysts. These issues are rarely addressed in current literature and require more in situ experimental evidence to verify.
4.4 Nitrogen Reduction Reaction (NRR)
The synthesis of ammonia by combining the environmentally enriched N2 molecule with H2O is an environmentally friendly method with low energy consumption, which is expected to replace the traditional Haber–Bosch method. However, significant challenges still exist in terms of the catalytic activity and selectivity of NRR catalysts due to the chemical inertness of N2 molecules, slow reaction kinetics, and competition with the HER. Furthermore, the current production level of ammonia through NRR is mainly at the microgram scale. It would pose obstacles for qualitative/quantitative measurements, requiring precise identification and differentiation of the actual catalytic products and contaminants.143 Electrocatalytic NRR is a six electron-proton coupled process that involves various reaction mechanisms, including the dissociative pathway, associative alternating pathway, associative distal pathway, and the possible lattice-N mechanism.144
To date, various types of active metal components have been reported to exhibit certain catalytic activity and selectivity for electrocatalytic NRR. Ma et al. prepared defect-rich PdZn nanoparticles anchored on the N-doped hollow carbon framework (etched-PdZn/NHCP) through the acid etching process of MOF-derived precursors (Figure 14A).145 In phosphate buffer solution, etched-PdZn/NHCP demonstrated a high NH3 yield of up to 5.28 μg mg−1cat and a FE of 16.9 % (Figure 14B). On this catalyst, the NRR reaction pathway follows the associative distal mechanism. The PdHx phase formed at the Pd sites interacts with dissolved N2 molecules, activating their triple bonds. The activated N2 undergoes consecutive protonation processes at the distal N, eventually releasing the first NH3 molecule. Subsequently, consecutive protonation occurs at the proximal N, leading to the formation of the second NH3 molecule (Figure 14C). The excellent catalytic activity is attributed to the highly exposed PdZn nanoparticles with rich defects, as well as the porous carbon framework for faster mass transfer (Figure 14D). In addition to Pd, noble metal Ru-based components, such as single atoms146 and nanoparticles,147 have also been reported to exhibit respectable NRR catalytic activity and selectivity. It is worth noting that, due to the high responsiveness of Pt-group metals to HER, more precise modulation of the structure, composition, and electronic structure is required when designing active sites in noble metal-based NRR catalysts.
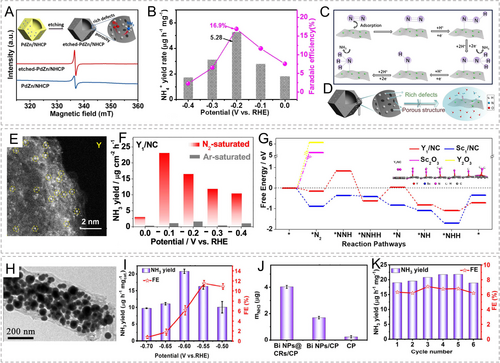
(A) Electron paramagnetic resonance (EPR) spectra of pristine and etched PdZn/NHCP; (B) NH3 yield rates and FEs of etched-PdZn/NHCP at different potentials; (C) NRR pathway and (D) reaction illustration on etched-PdZn/NHCP. Reproduced with permission.145 Copyright 2020, Elsevier. (E) STEM image of Y1/NC; (F) NH3 yields of Y1/NC at different potentials; (G) NRR free energy diagram on Y1/NC and Sc1/NC models. Reproduced with permission.148 Copyright 2020, American Chemical Society. (H) TEM image of Bi NPs@CRs; (I) NH3 yields and FEs of Bi NPs@CRs/CP at different potentials; (J) NH3 amount with different catalysts at −0.60 V vs. RHE; (K) circulating tests of Bi NPs@CRs/CP at −0.60 V. Reproduced with permission.153 Copyright 2021, American Chemical Society.
Rare earth metal elements have also been developed for electrochemical NRR processes. Liu et al. obtained single-atom Y1/NC and Sc1/NC catalysts modulated by N and C atoms, through the pyrolysis of Y- and Sc-doped ZIF-8 (Figure 14E).148 Y and Sc atoms coordinated with six nitrogen/carbon atoms to form unique coordination structures. Therefore, in acidic media, Y1/NC exhibited superior NRR catalytic activity, achieving an NH3 yield of 21.8 μg cm−2 h−1 and a FE of 12.1 % at −0.1 V vs. RHE (Figure 14F). That is a level that cannot be achieved by rare earth metal oxide catalysts. Similarly, the Y1/NC catalyst also followed the associative distal pathway, which exhibits a relatively low energy barrier towards NRR (Figure 14G).
Other cheap and plentiful transition metals can also be utilized as effective NRR catalysts. Transition metal-based catalysts (such as Fe, Co, Ni), have been extensively developed as catalysts for electrocatalytic NRR.149 Liu′s group successfully synthesized single-atom Fe sites on N-doped carbon polyhedrons.150 In a neutral aqueous solution at room temperature, this catalyst exhibited a high NH3 production rate of 62.9±2.7 μg h−1 mgcat.−1 and a FE of 18.6±0.8 %. X-ray absorption fine structure analysis and theoretical calculations revealed that the single-atom Fe sites were stabilized in the Fe−N4 configuration, facilitating the activation of N2 molecules. Zhang et al. developed Mo−Co bimetallic nanoparticles by combining strongly adsorbed Mo with weakly adsorbed Co on ZIF-derived carbon (Mo−Co/NC).151 In 0.1 M Na2SO4 solution, this catalyst demonstrated an ammonia production rate of 89.8 μmol h−1 gcat.−1 and a FE of 13.5 %. Additionally, the bismuth-based active component is also used to catalyze NRR due to its catalytic inertia to HER.152 Wang et al. stabilized bismuth nanoparticles in carbon rods derived from MOFs (Bi NPs@CRs) (Figure 14H).153 In acidic media, Bi NPs@CRs exhibited a high ammonia yield of 20.80 μg h−1 mgcat.−1 and a FE of 11.50 % (Figure 14I, J). Furthermore, this catalyst demonstrated excellent electrochemical durability (Figure 14K).
It is not difficult to see that the application bottlenecks of MOF-derived carbon-supported metal catalysts to drive electrocatalytic NRR are still the low NH3 yields and FEs. The competition with HER inevitably leads to the limited selection of active metal components, and the problem of external disturbance puts higher requirements on the quantitative detection of products. The dynamic evolution and catalytic mechanisms of different catalytic centers are still unclear and have not been thoroughly studied. In addition, Li-mediated NRR catalysis in organic electrolytes may have better research prospects, but the application of MOF-derived carbon-supported metal catalysts in Li-mediated NRR has not been reported yet.
4.5 Nitrogen Oxide Reduction Reaction (NOxRR)
Nitrogen oxide (NOx) is an important class of pollutants or greenhouse gases generated in industries such as chemical and power generation. It mainly consists of liquid nitrogen oxyanions (NO3−, NO2−) and gaseous pollutants (NO, NO2, N2O). Recently, there has been increasing research on the NOxRR due to their thermodynamic and kinetic advantages over NRR.154
In the realm of nitrogen oxyanion electroreduction, current research predominantly focuses on nitrate electroreduction, with ammonia being the primary product.155 Zhang et al. effectively drove NO3−RR using Co-doped Fe@Fe2O3 (Co−Fe@Fe2O3) embedded in a porous graphitic carbon framework derived from MOF-74.156 The carbon-based framework exhibited a porous flower-like nanosphere morphology, with the graphitic carbon providing excellent encapsulation of the internal metal particles (Figure 15A, B). The metal components consisted of a mixed phase of Fe2O3 and Fe, with Co atoms incorporated as dopants within these metal components (Figure 15C, D). According to the results of chronoamperometry and ultraviolet-visible (UV/Vis) analysis, in a neutral electrolyte containing NO3−, the catalyst continuously converted NO3− into NH3 products, exhibiting 96.7±0.2 % nitrate removal and 99.0±0.1 % NH3 selectivity after 10 hours (Figure 15E). In the linear sweep voltammetry (LSV) curve, Co−Fe@Fe2O3 exhibited an onset potential of −0.65 V vs. RHE, demonstrating significant NO3−RR catalytic activity. At −0.95 V vs. RHE, it achieved the highest NH3 yield of 1505.9±130.5 μg h−1 cm−2 (Figure 15F). DFT calculations indicated that Co−Fe@Fe2O3 possessed an optimal *NO3− adsorption strength, facilitating the adsorption/desorption process. Through an eight-electron sequential deoxygenation (*NO3−→*NO2−→*NO→*N) and hydrogenation process (*N→*NH→*NH2→*NH3), Co−Fe@Fe2O3 exhibited a low energy barrier of only 0.18 eV in its rate-determining step (*N→*NH), which was significantly lower than those of the undoped Fe@Fe2O3 and pure-phase Co (Figure 15G). The doping of Co adjusted the d-band center of Fe, thereby modulating the adsorption strength of intermediates and suppressing the generation of hydrogen competitors. Song et al. developed a catalyst for NO3−RR at ultra-low NO3− concentrations, primarily based on Cu nanoparticles encapsulated in a MOF-derived porous carbon framework.157 At −0.3 V vs. RHE, Cu@C achieved a FE of 72.0 %. And at −0.9 V vs. RHE, the NH3 production rate reached 469.5 μg h−1 cm−2. Such performance is highly remarkable for such a low NO3− concentration of 1×10−3 M.
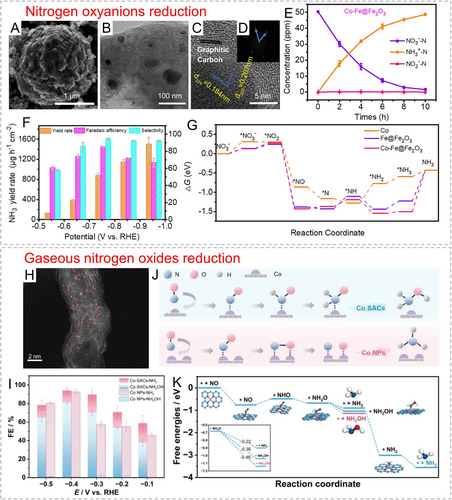
(A) SEM, (B) TEM, (C) high-resolution TEM and (D) fast-Fourier-transform (FFT) images of Co−Fe@Fe2O3; (E) concentration change with time for NO3−, NO2−, and NH3 on Co−Fe@Fe2O3; (F) FE, NH3 yield, and NH3 selectivity on Co−Fe@Fe2O3 at different potentials; (G) NO3−RR free energy diagram of different models. Reproduced with permission.156 Copyright 2021, National Academy of Sciences. (H) STEM images of Co SACs; (I) FEs of NH3 and NH2OH at different potentials; (J) NORR pathway on Co SAs and Co NPs for the generation of NH2OH and NH3, respectively; (K) NORR free energy diagram to gain NH2OH and NH3 on Co SACs catalyst. Reproduced with permission.161 Copyright 2023, Wiley-VCH.
Nitrite (NO2−) is also an intermediate in NO3−RR and has received some attention.158 However, the direct application of MOF-derived carbon-supported metal catalysts in this area is relatively limited, requiring further exploration in the future.
In the case of gaseous nitrogen oxides electroreduction, the most common process is nitric oxide reduction reaction (NORR), whose products include NH3, NH2OH, etc.159 Zhang et al. anchored single-atom Ce sites on MOF-derived nitrogen-doped hollow carbon spheres.160 In acidic electrolyte, it could efficiently electrochemically reduce NO to NH3 with a maximum FE of 91 % and achieve a high NH3 production rate of 1023 μg h−1 mgcat.−1. The Ce−N4 configuration effectively activated NO molecules and lowered the reaction barrier for the transition from *NO to *NOH intermediates, thereby enhancing the NORR catalytic activity. Zhou et al. demonstrated Co single atoms and nanoparticles anchored on carbon framework derived from ZIF-67 (Figure 15H).161 In phosphate buffer solution, the introduction of NO effectively lowered the onset potential of both catalysts and significantly enhanced the activity, indicating a favorable kinetic of NORR. However, they exhibited selectivity towards different products. The NORR product with Co nanoparticles was predominantly NH3, while Co single atoms selectively reduced NO to NH2OH. For Co single atoms, the highest FE and NH2OH yield were 81.3 % and 406.6 μmol mgcat−1 h−1 at −0.4 V vs. RHE, respectively (Figure 15I). Computational results for the reaction pathway also indicated that Co single atoms favored the formation of NH2OH products over NH3 products (Figure 15J, K).
Recently, the development of NOxRR catalysts has become a hot topic in the field of electrocatalysis. Compared to NRR, NOxRR has higher yields and FEs, thus attracting much attention. Currently, research on the NOxRR catalysis with MOF derivatives mainly focuses on the selective production of different products in different application scenarios, in order to provide various catalyst candidates. However, the evolution of catalytic centers, efficient catalysis in practical environments, and the design of integrated systems have not been extensively studied in recent literature.
5 Summary and Perspective
In this review, the modulation strategies of three core components of MOFs, namely organic ligands, metal nodes, and various modifiers, have been well summarized. Based on these three components, we have conducted a systematic analysis of the morphological control, compositional modification, defect structure, and controllable metal loading of MOF-derived carbon-supported metal materials. The well-developed pore structure provides an ideal pathway for mass and electron transfer processes and exposes active metal species. The highly tunable composition meets the different requirements of various applications and ensures high selectivity of catalysts. Furthermore, the regulation of electronic structure on catalytic centers due to strong metal-support interactions can significantly optimize the intrinsic catalytic activity. As a result, MOF-derived carbon-supported metal catalysts have been widely used in various electroreduction applications. The structural modulation, catalytic performance, and feasible catalytic mechanism of such catalysts have been effectively elucidated based on recent reports. Despite the significant progress made in this field, there are still many aspects that require further in-depth research.
-
Deep principles of MOF structure modulation. In synthesis, different dimensions of MOF materials can be obtained by selecting different ligands, modifiers, reaction conditions, etc. Some articles have also provided possible explanations for specific structure formation of MOFs. However, it is a very interesting and meaningful research goal to build a MOF library with different dimensions, by customizing the MOF crystal growth based on the specific properties of SBUs, ligands, and modifiers. Currently, high-throughput materials computation empowered by artificial intelligence has provided screening strategies for many fields, which can also be used for predicting and selecting architectures in the MOF crystal growth.
-
Differentiation between exchange and impregnation strategies. For MOF precursors, whether ligand exchange or metal node exchange, they need to be strictly distinguished from the wet impregnation process mainly dominated by pore confinement and surface adsorption. In many literatures, concepts are often misused. The exchange process might change the crystal structure and morphology of MOFs, while the impregnation process always retains the original crystal structure. In addition, spectroscopic techniques can effectively monitor the coordination structures of ligands and metals, which are powerful means to identify the difference between these two.
-
Precise modulation of the electronic structure for active metal species. Different electroreduction reactions require different active metal components. In fact, transition metal-based catalysts have been used in many competitive electroreduction reactions in previous studies. Although the morphology and metal phase are similar, more precise modulation of the electronic structure of catalytic centers is required for different catalytic reactions, which significantly affects the adsorption-desorption behavior of different intermediates and thus changes reaction selectivity. However, in many studies, the question of how controllable metal loading strategies change the electronic structure of active metal species is often described phenomenologically without providing a more reasonable explanation.
-
Dynamic evolution of active site configuration. Although MOF-derived carbon-supported metal materials typically maintain relative stability in phase and structure under electroreduction potentials, the chemical instability of some metal components in different electrolytes needs to be treated with caution. Therefore, understanding the dynamic processes of metal components during electroreduction, especially the evolution of catalytic active centers, can obtain more accurate catalytic site information, which is a cutting-edge and challenging research area.
-
Examination of reaction mechanisms. Even the relatively simple two-electron HER reaction has reaction paths for different elementary reactions (Volmer, Heyrovsky, and Tafel steps), not to mention complex multi-proton-electron coupled processes. In the electric double layer carrying the solid–liquid–gas three-phase interface, the different distribution situations of electrolyte molecules and ions can also affect the adsorption configuration of key intermediates. Therefore, with the assistance of theoretical simulations and spectroscopic techniques, a more systematic study on the catalytic mechanisms of electroreduction reactions is highly meaningful.
-
Although many MOF-derived catalysts have shown decent overall catalytic performance in lab-level tests, their demonstration in practical industrial conditions is often ignored. Under practical application conditions, factors such as reaction temperature, applied voltage, use of membrane electrode assembly, and different mass/charge transfer processes always lead to discrepant catalytic performance. Therefore, the catalytic activity, selectivity, and durability of MOF-derived carbon-supported metal catalysts must be evaluated under application conditions in the practical devices. In addition, the structure, composition, and material phase of these catalysts under lab-level tests and actual operating conditions need to be deeply explored through ex situ/in situ characterization means, in order to analyze the failure mechanism of catalysts and provide guidance for the future rational design.
Undoubtedly, MOF-derived carbon-supported metal materials possess significant advantages that many other materials lack, and can be applied in various energy-related applications. The rational modulation of precursor composition and active center is the prerequisite for obtaining highly active, selective, and durable catalysts. Given the promising prospects of MOF-derived carbon-supported metal electrocatalysts, we do hope that this article can provide some enlightenment for designing and constructing related catalysts with specific morphological features, different metal-based catalytic centers, and favorable reaction pathways.
Acknowledgments
J. Zhu acknowledges the funding support from Hong Kong Innovation and Technology Commission via the Hong Kong Branch of National Precious Metals Material Engineering Research Center.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Biographical Information
Jiawei Zhu obtained his M.S. (2019) and Ph.D. (2023) degrees from Wuhan University of Technology under the supervision of Prof. Shichun Mu. He is currently a postdoctoral researcher in City University of Hong Kong under the supervision of Prof. Xiong Wen (David) Lou. His research interests focus on the rational design and construction of defective carbon-based and noble-metal based nanomaterials towards electrocatalytic applications.
Biographical Information
Dr. Xue Feng Lu is currently a full professor at the State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, China. He obtained his Ph.D. degree from Sun Yat-sen University in 2017. Subsequently, Dr. Lu carried out his five-year postdoctoral research at Nanyang Technological University, Singapore, and The Hong Kong Polytechnic University. His research interests focus on hydrogen and oxygen electrocatalysis towards sustainable energy storage and conversion.
Biographical Information
Xiong Wen (David) Lou received his B.Eng. (first-class honors) (2002) and M.Eng. (2004) degrees from the National University of Singapore. He obtained his Ph.D. degree in chemical engineering from Cornell University in 2008. He is currently a Chair Professor in Materials Chemistry in City University of Hong Kong. His current research is focused on the design and synthesis of nanostructured materials for different applications in batteries, electrocatalysis, and photocatalysis.







