Zinc ion Batteries: Bridging the Gap from Academia to Industry for Grid-Scale Energy Storage
Abstract
Zinc ion batteries (ZIBs) exhibit significant promise in the next generation of grid-scale energy storage systems owing to their safety, relatively high volumetric energy density, and low production cost. Despite substantial advancements in ZIBs, a comprehensive evaluation of critical parameters impacting their practical energy density (Epractical) and calendar life is lacking. Hence, we suggest using formulation-based study as a scientific tool to accurately calculate the cell-level energy density and predict the cycling life of ZIBs. By combining all key battery parameters, such as the capacity ratio of negative to positive electrode (N/P), into one formula, we assess their impact on Epractical. When all parameters are optimized, we urge to achieve the theoretical capacity for a high Epractical. Furthermore, we propose a formulation that correlates the N/P and Coulombic efficiency of ZIBs for predicting their calendar life. Finally, we offer a comprehensive overview of current advancements in ZIBs, covering cathode and anode, along with practical evaluations. This Minireview outlines specific goals, suggests future research directions, and sketches prospects for designing efficient and high-performing ZIBs. It aims at bridging the gap from academia to industry for grid-scale energy storage.
1 Introduction
Battery technologies for grid-scale energy storage have emerged as critical components in addressing the intermittency and variability of renewable energy sources, such as solar, wind, hydropower, etc. Among these technologies, lithium-ion batteries (LIBs) and lead-acid batteries (LABs) have dominated the market due to their widespread use and impressive performance.1 However, the growing demands for cleaner and more efficient energy storage solutions, are driving us to explore alternative options. Zinc ion batteries (ZIBs) that use Zn metal as anode have emerged as promising candidates in the race to develop practical and cost-effective grid-scale energy storage systems.2 ZIBs have potential to rival and even surpass LIBs and LABs for grid scale energy storage in two key aspects: i) earth abundance of Zn, ensuring a stable and affordable raw material source, and ii) high safety that is associated with the utilization of aqueous-based electrolytes and non-toxic Zn metal anode.3 While LIBs hold great advantages in terms of their high working voltage and low self-discharge rate, the improvements for overall performance of ZIBs rely heavily on intensive studies.
Research has made significant strides in improving ZIBs’ performances, but transitioning from small-scale prototypes to large-scale, commercially viable energy storage systems remains a challenge.4 The main issue is the neglect of ZIBs′ limitations in terms of real energy density and calendar life. While academia has made substantial progress, there is a pressing need to bridge gaps between fundamental research and practical implementation. To achieve the practical implementation of ZIBs for grid-scale energy storage, two critical factors must be addressed. Firstly, the real energy density based on the full battery pack is not fully illustrated in ZIBs owing to the excess use of battery components, such as electrolyte and anode. Secondly, predicting the cycling life of ZIBs with a reliable calculation method is challenging, given the capacity loss from both the cathode and anode. As a result, the translation of these laboratory outcomes into real-world scenarios remains uncertain.
In this paper, we will explore these critical gaps between research and industry that exist in the ZIBs, and shed light on the parameter paths and goals essential for a successful transition from academia to industry. Specifically, we focus on achieving high practical energy density based on the whole cell weight and long calendar life referring to the operational lifespan of a battery in terms of time, regardless of the number of charge–discharge cycles.
We employ a formula-based approach to assess the factors hindering practical energy density and propose a reasonable correlation for predicting the calendar life of ZIBs. The formulas offer several advantages: 1) comprehensive consideration of various factors affecting energy density and active material weight, aiding precise calculations, and establishing solid research foundations. This includes quantitative insights into factors like N/P (capacity ratio of negative electrode to positive electrode) ratio, E/P (electrolyte to positive electrode in μL mg−1) ratio, electron transfer number, and the impact of energy utilization on the energy density of ZIBs. 2) A diagnostic formula using N/P ratio and Columbic efficiency (CE) to predict the cycling life for Zn anode. 3) Provision of guidelines for academic research, emphasizing techniques, electrolyte weight control, and optimizing electrode configuration in line with the formulated insights. 4) Identification of limitations in traditional cathode materials for reaching a high energy density at cell level for grid-scale energy storage. We consider the industrial benchmark of 150 Wh kg−1 reported for sodium-ion batteries,1a, 5 as a high energy density value for grid-scale energy storage. We are suggesting cathode alternatives in ZIBs, including iodine, sulfur or emerging materials for advancements in energy density. In our comprehensive exploration of essential factors for designing practical ZIBs technologies, we borrowed the descriptors from the design of commercial lithium-sulfur batteries, and introduced the use of two descriptors, and , as analytical tools.6 We present a clear and detailed formulation summarizing critical parameters crucial for achieving high-energy-density ZIBs.
Additionally, we engage in discussions regarding calculating the real calendar life rather than cycling numbers, as well as presenting the detailed gaps between current laboratory studies and industrial requirements. We also compared the energy density and CEs calculated from our formulations with data from existing literature on ZIBs, highlighting the gap between practical targets and current research. Finally, we outline our perspectives on the next steps, which include using realistic low current rates and studying self-discharge mechanisms, etc., in the development of ZIBs for grid-scale energy storage.
2 Criteria of Grid-Scale Storage
Before transitioning battery technologies to industrial production, it is important to meet comprehensive requirements. These requirements include: 1) ensuring a long calendar life exceeding 10 years under low current rates1a (equivalent to more than 3000 cycles at a low current rate of C/24); 2) maintaining a capacity retention rate above 80 %;1a 3) operating within a wide temperature range of −30 °C to 50 °C;1a 4) high safety with no combustibility or explosion risks.1a, 7 5) minimizing the self-discharge rate to less than 1 % per day;1a 6) keeping costs below $100 per kWh (Figure 1a, as proposed by the US department of energy (DOE) in 2022). While energy density may be a less concern for grid scale energy storage, a battery with a high cell-level energy density would make it more competitive for practical application. For example, sodium ion batteries were reported to reach 150 Wh kg−1, making them promising high-energy-density alternatives to LIBs that utilize LiFePO4 as a cathode5 for stationary energy storage. Regarding these characteristics (as seen in Figure 1b), ZIBs are gaining considerable attention as a promising energy storage solution. When compared to traditional LABs, ZIBs exhibit distinct advantages in most aspects, except the slight disadvantage in cost. While LIBs excel in high energy density, ZIBs offer a compelling safety and cost advantages for stationary energy storage applications, which prioritize low cost, long calendar life, wide temperature tolerance, and safety.
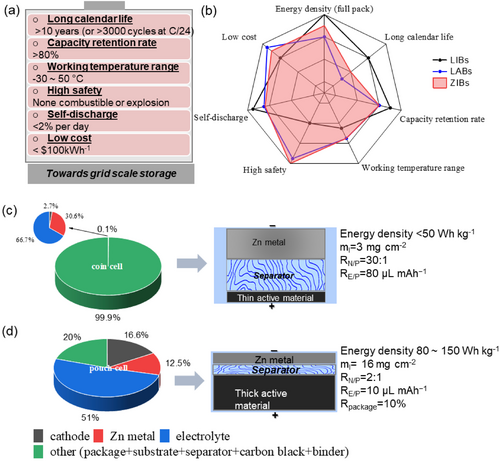
(a) Requirements for batteries towards grid-scale storage and (b) radar plot of advantages of ZIBs over the current mature LIBs and LABs. Pie charts comparison of the (c) coin cell and (d) pouch cell techniques using the MnO2 cathode as an example.
3 Technical Gaps between Academia and Industry Demand
3.1 To beat LIBs and LABs
As highlighted in Table 1, ZIBs demonstrate several advantages over both LIBs and LABs in terms of grid scale energy storage. The demand for cost-efficiency and enhanced safety in large-scale grid energy storage is driving the development of ZIBs, which hold promise of reducing costs to under $50 per kWh, putting them in direct competition with LIBs. Additionally, ZIBs exhibit a substantially higher energy density (eg. achieving 150 Wh kg−1 in Zn-MnO2 battery) than the commercial LABs (50 Wh kg−1), positioning ZIBs as a competitive choice for grid-scale applications.
Battery technology |
LIBs |
LABs |
ZIBs |
|---|---|---|---|
Safety |
Low |
High |
High |
Current cost (USD kWh−1) |
~1308 |
~508 |
|
Cost Goals (USD kWh−1) |
1008* |
358* |
|
Output voltage (V) |
1.8–2.21a |
0.8–1.812 |
|
Energy density (Wh kg−1) |
100–26513 |
30–5013 |
80–15014* |
Round trip efficiency |
|||
Daily self-discharge |
0.1 %–0.3 %13 |
0.1 %–0.3 %13 |
<1 %17 |
Service life |
5–15 years (mature)13 |
5–15 years (mature)13 |
15 year (predict based on the life of Zn anode)18* |
- * Data source: 1. DOE/OE-0034—Zinc Batteries Technology Strategy Assessment. 2. Energy Storage Grand Challenge Cost and Performance Assessment 2022 Foreword to 2022 Report. 3. “Technology”. Eos Energy Enterprises. Retrieved, 2023–05–14.
3.2 Technique gaps towards the goal
Previous efforts have focused on tackling issues associated with anode dendrites in ZIBs, however, many approaches use coin-cell configurations, flooded electrolytes dosage, and thick zinc foils. Coin cells are widely recognized in fundamental tests and assessing the electrochemical behaviours of materials, including voltage profiles, specific capacities, and electrochemical stability during initial exploration stages. However, their substantial diversity in experimental conditions poses a considerable challenge when attempting to compare and benchmark materials and concepts. Materials and concepts developed on the laboratory scale are typically studied under a range of experimental conditions that often differ significantly from the requirements of a large battery. Alternatively, pouch cells allow for scalability and accommodates diverse electrode loadings and geometries, enhancing represent ativeness for practical applications. Figure 1c and 1d illustrate the impact of cell configuration differences on energy densities, which can be significantly influenced across factors such as electrolyte dosage, cathode loading mass, and N/P ratios, and Zn thickness (areal capacity).19 Coin cells have limitations in low loading mass (<3 mg cm−2), flooded electrolyte (>80 μL mAh−1), and high N/P ratios (>30 : 1), which may not accurately represent practical electrode conditions.20 As a result, achieving a practical energy density of over 50 Wh kg−1 in a coin cell is challenging due to a significant portion of inactive materials, particularly the weight of the packaging (>99 %). In contrast, it is possible to regulate specific parameters in Zn-MnO2 pouch cells to reach the upper limit of energy density of 150 Wh kg−1, taking into account the total weight of the cell. To meet the desired criteria, it is crucial to control the package weight within 10 % of the total cell weight. Additionally, a high loading mass of cathode active material, such as 16 mg cm−2 for MnO2 cathode is required, along with an extreme N/P ratio of 2 : 1 under a lean electrolyte condition of 10 μL mAh−1.21 Notably, despite the incorporation of those specific design parameters, there is a lack of comprehensive quantitative analysis regarding how these parameters collectively influence cell-level energy density.22
4 Material Requirements
4.1 Formulating of practical energy density
4.1.1 Definitions:
: practical energy density based on the full pack of the battery combining energy loss from incomplete electrochemical reactions.
: practical energy density output based on the cathode active material only.
: theoretical energy density based on active cathode material only.
: energy utilization ratio combing energy loss from incomplete reactions.
: total mass percentage of both active cathode material and active Zn.
: number of Zn ions transferred into the cathode.
: specific capacity of active cathode material.
: theoretical specific capacity of active cathode material.
: output voltage of the cathode.
: theoretical voltage of the cathode.
: molar weight of active cathode material before electrochemical reacting with Zn ions.
: molar weight of active cathode material after incorporated with×of Zn ions.
: weight ratio of package materials in the whole battery.
: areal mass loading of active cathode material.
areal mass of substrate in the cathode based on a double side coating strategy.
: areal mass of separator.
: weight ratio active cathode material in the cathode, including host, conductive agent, and binder.
: electrolyte density.
: ratio of electrolyte to cathode in μL mg−1.
: ratio of areal theoretical capacity of negative and positive electrode.
4.2 Practical high active materials weight ratio Rweight
For illustrative purposes and to simplify calculations, we use commonly applied parameters found in real pouch cells.6 Specifically, the package weight ratio, denoted as , is set to be 10 wt %, referring to the accepted weight ratio in lithium-ion pouch cells. The thickness of the Ti foil is specified as 10 μm, with an areal density of 4.5 mg cm−2 (equivalent to 4.5 g cm−3). The areal density of the separator is taken from lithium-ion separator (1 mg cm−2). The anode is a highly conductive Zn metal foil. The density ( ) of the electrolyte is established as 1.138 g mL−1, determined from an aqueous 1 M ZnSO4. The parameter “ ” is set at 0.5 based on a one-electron reaction in the MnO2 cathode. The is calculated using a 1 : 1 weight ratio of 1 M ZnSO4 electrolyte to MnO2 cathode material. According to these parameters, we illustrate the correlation derived from the equation (3). As demonstrated in Figure 2a, we explore the relationship between and concerning while keeping the remaining key variable, , fixed at an extreme low value of 1 μl mg−1, as refereed from LIBs. It is evidenced that modifying two key parameters, and , has a significant impact on . A notable enhancement in is observed by decreasing from 10 : 1 to 1 : 1, resulting in an improvement from 10 % to 21.9 % under a maximum ml of 16 mg cm−2. In contrast, under a constant low of 1 : 1, increasing the loading mass ( ) from 2 to 16 mg cm−2 leads to an increment in from 14.6 % to 21.9 %.
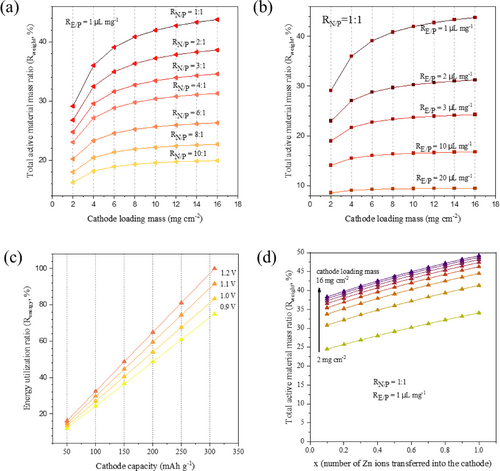
Formulation of and from key parameters using MnO2 as an example. Calculation of total mass ratio in full cell with different (a) (b) and cathode mass loading; (c) Calculation of energy utilization ratio with different capacity and voltage; (d) Calculation of total mass ratio with the change of number of electron transfer in the cathode.
The correlation between electrolyte amount and is shown in Figure 2b. While maintaining a low of 1 : 1, conversely, decreasing yields a more effective enhancement of . While keeping constant at 0.1 μL mg−1, increasing ml from 2 to 16 mg cm−2 almost double the , increasing from 17.1 % to 28.4 %. These demonstrations suggest that decreasing or can enhance compared to increasing mass loading . However, it is worth noting that achieving a lean electrolyte condition below 1 μl mg−1 (equals to 0.31 uL mAh−1 based on the aqueous 1M ZnSO4 electrolyte and MnO2 cathode) is currently impractical with the existing battery technique. As a result, it remains challenging to attain an beyond 21.9 % for the MnO2||Zn full battery in real-world conditions.
Equation (2) and Figure 2c demonstrate that increasing both the specific capacity of cathode ( ) and real voltage output ( ) improves energy utilization ( ). For example, increasing from 50 to 308 mAh g−1 (assuming =1.2 V) dramatically enhances the from 16 % to 100 %. Therefore, it is necessary to establish battery testing conditions that approaches the full theoretical capacity of the cathode to reach high . Based on the above discussion by controlling N/P and E/P ratios within a low range, it is evident that the 1e− redox in MnO2 to form Zn0.5MnO2 has reached the limit of 21.9 %, hindering its . To overcome this limitation, increasing to accommodate more Zn2+ can enhance the to 40 %, as displayed in Figure 2d.
4.3 Prediction of practical energy density
The relationship between the final battery energy density, and is shown in Figure 3a, as depicted by equation (1). Achieving a high of 100 % enables the to approach 150 Wh kg−1 when exceeds 40 %. However, according to calculations in Figure 2a and 2b, our current reports on energy density without controlling battery package weight typically fall below 70 Wh kg−1, with a low less than 43.8 %. As a result, based on these findings, the MnO2 faces challenges in reaching 150 Wh kg−1. Under optimal conditions with a maximum of 43.8 % (based on a high loading mass of 16 mg cm−2, low =1 : 1 and =1 μL mg−1) and at 100 %, the maximum can only reach 161.9 Wh kg−1. To address the challenge of low energy density, it is imperative to explore alternative cathode candidates.

Formulation of and practical using different active cathode materials. (a) Calculation of full-cell energy density ( ) with different and (Equation (1)). (b) Bar comparison of theoretical energy densities (grey) and maximum practical energy densities (blue) using different active cathode materials, where the PB (prussian blue), PANI and I2 (2e− redox) are unable to reach the theoretical value (shaded on each bar). The theoretical capacity of MnO2 is 308 mAh g−1 based on one molecular weight and 1e− transfer reaction. The maximum practical energy density only takes a simple consideration of active cathode, anode and electrolyte materials under a N/P ratio of 2 : 1 and lean electrolyte of 1 μl mg−1. (c) Practical range calculated with a wide cathode loading mass of 2 mg cm−2 to 16 mg cm−2 under 1 : 1 and 1 μL mg−1. The PB, PANI and I2 (2e− redox) are shaded as they are unable to achieve the practical . (d) Calculated minimum that requires to achieve 150 Wh kg−1 with different cathode materials, where the PB, PANI and I2 (2e− redox) are unable to reach 150 Wh kg−1.
Figure 3b compares the theoretical energy density of several representative cathode materials and the maximum . The latter considers only the weight of active cathode and anode materials, along with the electrolyte dosage, using low of 1 : 1 and of 1 μL mg−1. V2O5, I2 (based on 4e− redox) and sulfur show great potential to exhibit high energy density, benefiting from their relatively high theoretical energy density. To identify the existing gap in achieving a high practical energy density, we analyze additional battery components, including package, current collector, and separator. We calculate the minimum required to meet a high cell-level energy density of 150 Wh kg−1, as displayed in Figure 3c. Figure 3c also presents practical values across a broad cathode loading mass range (from 2 mg cm−2 to 16 mg cm−2) under 1 : 1 and 1 μL mg−1 conditions. The analysis indicates that minimum required for MnO2, V2O5, I2, sulfur is possible, as these values are fall within or below the practical range. It is noted that I2 (4e− redox) and sulfur hold great advantages in requiring low due to their high theoretical capacity.
Although reaching high is possible in ideal battery manufacturing, achieving the high theoretical capacity is difficult due to irreversible capacity loss in practical testing conditions. Factors such as temperature variations, material impurities, and structural flaws impede energy storage efficiency of the material. In this case, the minimum energy density required for the cathode ( ) in research is calculated based on optimal battery parameters (Figure 3d). To reach a high , such as 150 Wh kg−1, all listed cathode materials must deliver an energy density ( ) of over 300 Wh kg−1, except for sulfur, which requires a lower threshold of 246.3 Wh kg−1. Prussian blue (PB), polyaniline (PANI) and I2 (2e− redox) all fail short of meeting this goal for the cathode materials due to their low theoretical capacity. MnO2 and V2O5 are expected to achieve 342.6 and 344.7 Wh kg−1, respectively. These formulas can help establish meaningful benchmarks for laboratory research and guide the development of cathode materials in the future. With these validated equations, recommended battery parameters and promising cathode materials can be proposed for designing high-energy-density ZIBs batteries.
4.4 Coulombic efficiency goals towards long calendar life
As discussed, ZIBs show promising energy densities that can compete with LABs when coupled with high-capacity cathodes. To maintain 80–90% capacity retention after 1,000 cycles, the battery needs to have a high CE of over 99.98%, ideally exceeding 99.99%. Achieving this can be challenging due to capacity loss from both the cathode and anode. To accurately assess Zn reversibility, CE measurement should be conducted using a non-excess Zn working electrode (such as Cu or Ti foils) with Zn foil serving as a reservoir.23 Importantly, in Zn||Zn cells, using capacity/time control results in a constant CE of 100 %, which lacks practical significance. Asymmetric cells (e.g. Zn||Cu) offer a more direct approach to assess and compare Zn metal CE.
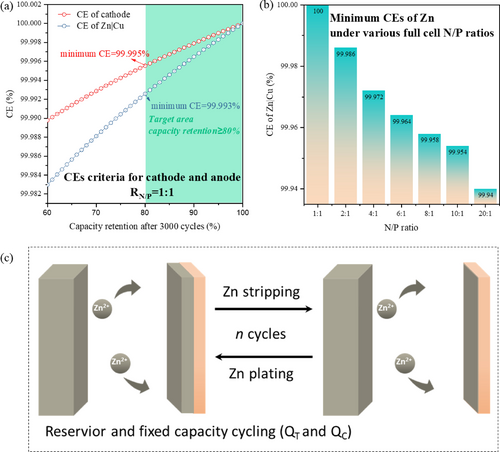
Formulating CEs results following 3000 cycles (based a current density of C/24). CEs with capacity retention rates for (a) Zn anode and cathode with a N/P ratio of 1 : 1. (b) minimum CEs for Zn and N/P ratios of Zn anode and cathode. (c) Illustration of “Zn reservoir” CE testing protocols.
However, achieving these CE objectives for both Zn anode and cathode material in practical batteries is challenging, requiring consideration of irreversible capacity losses on both electrodes and electrolyte. Typically, the evaluation of cathode CE takes priority over the full battery, and the anode-free batteries using a fixed capacity of the anode (Figure 4c), emerges as an ideal approach for assessing the true CE of active cathode materials.
5 Perspective and Summary
5.1 Cathode materials development
As formulated in Equation (1), (2) and (3), the progression of ZIBs towards a high energy density currently faces dilemmas centered around identifying cathode materials that satisfy several key criteria: 1) High discharge capacity; 2) Elevated working voltage; 3) Structural and crystallographic traits conducive to accommodating Zn2+ ions and enhancing typically slow diffusion kinetics; 4) Robust crystal structures capable of withstanding extensive cycling; 5) Economic feasibility and environmental consciousness. Unfortunately, existing materials do not fully align with these specifications, raising concerns about achieving the goals in energy density and cycling life. Cathode materials based on Mn and V chemistry play a key role in the development history of ZIBs and can meet the minimum to achieve 150 Wh kg−1 owing to their high theoretical energy densities (Figure 3c). Although many promising cathode materials, such as MnO2, V2O5, and I2 have been extensively studied, their performance with zinc anodes is not always satisfactory due to dissolution and side reactions in aqueous electrolytes. In addition, improving electrode preparation techniques to increase the loading mass of active material or reduce side reactions with aqueous electrolytes can help enhance the energy density.2b
Specifically, significant dissolution of Mn2+ was observed in Zn||MnO2 batteries due to the Jahn–Teller effect in MnO2, adversely impacting reversibility and cycling performance. Various solutions have been proposed, including pre-adding Mn2+ salts, controlling pH values, engineering solvation structure of electrolytes, and developing an artificial solid electrolyte interphase (SEI) layer on electrode surface.2c, 25 Nevertheless, these approaches pose additional costs for electrolytes and Mn-based cathodes, raising concerns about the cost of practical applications. Vanadium based cathodes also face structural degradation and active materials dissolution.26 Progress has been made in enhancing stability at high currents exceeding 1 A g−1 via materials structure design. This includes various modification strategies, such as using electrolyte modification (including additives, high concentration systems, hydrogels, etc.27), introducing guest species between cathode material layers for a “pillar effect”,28 including oxygen vacancies (eg. NH4V4O10), or modifying natrium super ionic conductor type cathode (eg. Na3V2(PO4)3).29 While NH4V4O10 and Na3V2(PO4)3) offer great advantages in terms of high voltage operation and ionic conductivity, their widespread deployment faces challenges in scalability and cost-effective production. Simultaneously meeting all requirements is difficult. Practical approaches should include electrolyte design and exploration of new cathode materials and storage mechanisms.30
Besides Zn2+ intercalation or conversion, higher capacity can be achieved by utilizing H+ storage, or H+/Zn2+ co-storage.31 However, challenges remain in utilizing proton storage due to their influence on irreversible cathode dissolution under small current rates.2b Therefore, feasible host materials, such as imine compounds31 and S-MoO232 that can carry out a simultaneous H+ and Zn2+ insertion/extraction process with enhanced performance should be considered and developed. In addition to proton storage, the optimal choices for achieving high-capacity cathode materials are narrowed down to sulfur and I2 (with 4e- redox). However, unlike the intercalation/deintercalation of zinc ions in layered cathode materials, these conversion cathodes present a unique challenge. The solid-to-liquid transition in sulfur and I2 cathodes results in the shuttling effect of polysulfides and polyiodides, adding complexity to their performance enhancement. Recent reports have partially mitigated the shuttling effect in some cases by anchoring polysulfides or polyiodides through electrolyte design. However, numerous challenges persist for practical applications. For instance, the cycle stability of Zn−S batteries currently poses a bottleneck, and increasing the loading of I2 remains a challenge.
From the cathode material perspective, the path to increasing capacities seems to lean towards leveraging multi-electron redox activity and integrating additional redox ion pairs. As a result, their achievable appear exceedingly limited compared to the sulfur and I2. We believe that for materials with limited theoretical energy densities or those that are already approaching theoretical values, it is important to prioritize understanding their energy storage mechanisms and enhancing cycling stability.
5.2 Zn anode reversibility measurement
Measuring Zn reversibility significantly benefits from the use of asymmetric cells. However, zinc CE measurements are influenced by various variables, resulting in varied values reported in the literature, even for identical cell configurations. It is important to note that crucial factors, including current density, electrolyte volume, and Zn thickness, exert a significant influence on CE values. Given side reactions on metallic Zn side in aqueous electrolytes, we recommend initiating testing at lower current densities, such as 0.5 or 1 mA cm−2, with a capacity of 1 mAh cm−2. Increasing the applied current density can lead to higher overpotentials, particularly in unmodified bare cells. As a reuslt, careful consideration of Zn electrode parameters, including accurate thickness, surface polishing, and potential trade-offs, is essential. The use of excessively thick Zn foil and a flooded electrolyte is not recommended.33 In order to get an optimized performance, we suggest using a 50-μm-thick Zn foil with a 50 μL electrolyte, which adequately infiltrates the entire cells. Assuming an electrolyte density is 1.0 g cm−3 and an electrode area is 1.27 cm2 (diameter is 12.7 mm). As an example, a Zn (50 μm)//V2O5 (4 mAh cm−2) cell can be assembled to validate new ideas.
In addition to using the asymmetric cell, we suggest an alternative approach to practically assess Zn reversibility, known as the “reservoir” method34 in LIBs, to practically assess the average CE, as illustrated in Figure 4c. Specifically, Zn reservoir (QT) is plating on the electrode, while the battery experiences continuous cycles of stripping/plating at a consistent capacity (QC) below the initial Zn reservoir (QC<QT). As the cycling proceeds, side reactions progressively consume the Zn reservoir. After cycling, the Zn reservoir becomes entirely depleted, evident by the abrupt surge in the stripping potential.
5.3 Realistic evaluation process
To ensure their reliability, efficiency, and ability to address renewable energy intermittency in a rapidly evolving energy landscape, it is urgently demanded for building a scientific evaluating process for ZIBs. This evaluation should include several key aspects, including energy density, calendar life, and practical assessment, each playing a crucial role in determining the suitability of batteries for grid-scale applications, as displayed in Figure 5.
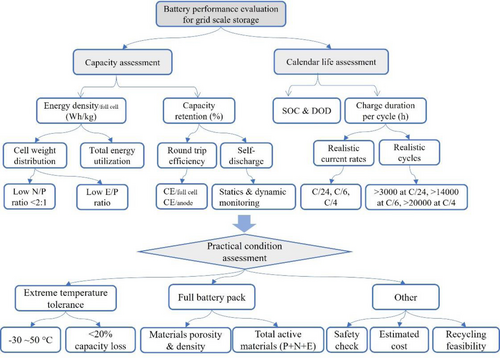
Evaluating process for practical grid scale energy storage applications of ZIBs. This includes the capacity and calendar life assessments, following with practical condition consideration.
The practical energy density of a battery is directly influenced by the physical size and weight of the battery package. The detailed distribution of cell weight can impact the overall energy density. Optimizing weight distribution is essential to maximize energy storage while maintaining structural integrity, a process that can be standardized with a calculation tool (Equation (3)). Apart from reporting a single energy density at a specific cycle number, capacity retention provides direct information into the capacity decay rate of the battery over cycling. Ensuing high-capacity retention of over 80% after thousands of charge–discharge cycles and achieving a low self-discharge rate are imperative for grid-scale applications. However, minimizing self-discharges in aqueous electrolytes poses a great challenge, and future advancements depend heavily on an in-depth study of underlying mechanisms.
For grid-scale storage, evaluating the calendar life of batteries is crucial, but it can be time-consuming. As a result, using a reasonable diagnosis formula (Equation (5) and (6)) to predict cycling life based on the average CE under specific current densities can expedite the experimental process. Effective management of the state of charge (SOC) and the depth of discharge (DOD) within reasonable values during charging/discharging is essential for achieving long calendar life. Avoiding prolonged and high SOC helps prevent degradation and increases the overall lifespan of the battery. Additionally, grid-scale storage systems must accomodate the intermittent nature of renewable resources, typically lasting 4–6 hours. Therefore, adopting small current densities of C/4, C/6, or even C/24 is necessary. However, current research is using much higher rates over 5C35 to avoid side reactions of active cathode materials with electrolytes. Realistically, achieving over 3,000 cycles at C/24, more than 14,000 cycles at C/6, and in excess of 20,000 cycles at C/4 is essential for a potential industrial battery system, which is still challenging forr current ZIBs chemistry.
Practical assessment considers various factors influencing real performance of batteries, including temperature adaptability. Evaluating capacity decay under extreme temperatures is important, as it significantly impacts the practical output ( and ) in real environments. As a result, calculating under different temperatures, alongside reporting specific capacity, provides a clear understanding of performance under extreme conditions compared to room temperature. Pouch cells, offering flexibility and scalability, are a preferred choice for grid-scale applications. Parameters, including package weight and electrolyte/cathode/anode ratios, can be optimized using the discussed equations. Assessing battery performance in full pouch configurations, representative of actual installations, ensures results align with practical implementation. In addition to performances, future designs should also minimize the risk of thermal runaway, fires, or explosions, ensuring system and environmental safety with the use of low-risk battery components. Furthermore, cost-effectiveness is critical for grid-scale storage, impacting the feasibility and economics of large-scale deployments. Evaluating the primary production cost of all battery materials, especially electrolytes that occupy most weight of the battery, is essential for overall project viability. For sustainability, assessing the feasibility of battery recycling is crucial, promoting a circular economy by designing materials with recycling in mind to reduce environmental impact.
5.4 Summary
This Minireview emphasizes the significance of considering two factors, denoted as and , beyond normal cathode specific capacity, to bridge the energy density gap between battery research and industrial demand. It highlights the correction of cycling life with N/P ratios to expedite the research-industry transition. Specifically, we offer a comprehensive set of formulas that consider various influencing factors impacting energy density and the weight of active materials. These formulas serve as valuable scientific tools for precise calculations. By quantifying the impact of each factor on energy density, especially considering factors like N/P ratio, E/P ratio, electron transfer number, and energy utilizations, this study provides critical insights into how to enhance energy density via cathode optimization. In addition to calculating practical energy densities, we will also emphasize diagnostics to expedite the calendar life of Zn anodes using the CE formulas under different N/P ratios, assuming 100% CE in the cathode. Furthermore, more attention should be given to the degradation of cathodes caused by various dissolution mechanisms under aqueous solutions to make a more accurate prediction.
The study provides essential guidelines for academic research, particularly in the construction and evaluation of full ZIBs. It emphasizes the improvement of techniques, with a specific focus on controlling electrolyte weight and optimizing electrode configurations. This Perspective opens avenues for future exploration in potential cathode materials and highlights existing limitations in traditional cathodes achieving the high practical energy density. It proposes exploring high energy density alternatives, such as I2 and sulfur, paving the way for advancements in energy storage technology. Through a holistic approach, this formula-intensive study provides us clear pathways toward the valuable research endeavour aimed at boosting practical integration of ZIBs into grid-scale energy storage.
Acknowledgments
This work was financially supported by Australian Research Council Discovery Projects (DP200101862, FL210100050, DE240100159), and an AINSE early career researcher grant (AINSE-ECRG, S.L Liu) Open Access publishing facilitated by The University of Adelaide, as part of the Wiley - The University of Adelaide agreement via the Council of Australian University Librarians.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Biographical Information
Professor Chaofeng Zhang is currently affiliated with the Institutes of Physical Science and Information Technology at Anhui University in Hefei, China. He obtained his B.Sc. from Lanzhou University and M.Sc. from Fudan University prior to completing his Ph.D. degree in 2013 at the University of Wollongong, Australia. Furthermore, he gained experience as a post-doctoral researcher at the National Institute of Advanced Industrial Science and Technology (AIST) in Japan. His research primarily focuses on electrochemistry for batteries, with specific emphasis on organic battery materials and electrolytes utilized in aqueous zinc-ion batteries.
Biographical Information
Dr. Shilin Zhang is currently serving as a Research Fellow at the University of Adelaide, Adelaide, Australia. He earned his Ph.D. degree from the Institute of Superconducting and Electronic Materials at the University of Wollongong, Australia in 2020. Prior to this, he completed his M.Sc. degree in 2016 at Beijing University of Chemical Technology. His current research is focused on the design, synthesis, and characterization of materials in the field of electrochemical storage devices.
Biographical Information
Professor Zaiping Guo is a Fellow of Australian Academy of Science, a Fellow of the Australian Academy of Technology and Engineering, an Australian Laureate Fellow at School of Chemical Engineering, The University of Adelaide, Australia. Her research focuses on the design and application of electrode materials and electrolyte for energy storage and conversion. Her research achievements have been recognized through numerous awards, including ARC Queen Elizabeth II Fellowship (2010), Future Professorial Fellowship (2015), Laureate Fellowship (2021), and the Clarivate Highly Cited Researcher (2018–2023).







