π-Radical Cascade to a Chiral Saddle-Shaped Peropyrene
Abstract
Reactions of open-shell molecular graphene fragments are typically thought of as undesired decomposition processes because they lead to the loss of desired features like π-magnetism. Oxidative dimerization of phenalenyl to peropyrene shows, however, that these transformations hold promise as a synthetic tool for making complex structures via formation of multiple bonds and rings in a single step. Here, we explore the feasibility of using this “undesired” reaction of phenalenyl to build up strain and provide access to non-planar polycyclic aromatic hydrocarbons. To this end, we designed and synthesized a biradical system with two phenalenyl units linked via a biphenylene backbone. The design facilitates an intramolecular cascade reaction to a helically twisted saddle-shaped product, where the key transformations—ring-closure and ring-fusion—occur within one reaction. The negative curvature of the final peropyrene product, induced by the formed eight-membered ring, was confirmed by single-crystal X-ray diffraction analysis and the helical twist was validated via resolution of the product's enantiomers that display circularly polarized luminescence and high configurational stability.
Phenalenyl1 (PLY)—the smallest molecular fragment of graphene that hosts an unpaired electron—decomposes in air or in the presence of an oxidant to a fused dimeric product, peropyrene (PP; Figure 1). This five-step π-radical cascade is a rare case of a well-understood decomposition pathway2, 3 of triangular graphene fragments,4 compounds that are primarily explored for their π-magnetism5-9 and as building blocks10, 11 of quantum materials. But is this transformation, in which two σ-bonds, one π-bond, and a new ring are formed in a single reaction, destined to carry the label2 “decomposition” or is it time to change this paradigm and embrace this kind of reactions as synthetically useful?
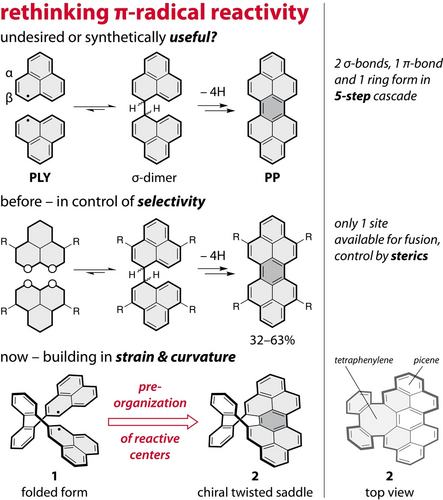
Let us rethink the π-radical cascade of phenalenyl and look at this typically undesired process through the lens of methodology. The unpaired π-electron in PLY is uniformly delocalized over the six α-positions,12 and even though this provides partial thermodynamic stabilization, PLY undergoes facile oxidative dimerization. The first step of this process is the reversible formation of a σ-dimer13 (Figure 1, top), which can occur at each α-position with equal probability. To suppress the reactivity of PLY, we must either increase its thermodynamic stability, as in 1,9-dithio-PLY,14 or provide sufficient kinetic stabilization, which can be achieved15, 16 by installation of bulky substituents that block all reactive positions. For example, three tert-butyl groups placed at the β-positions of PLY shield all α-positions sterically and fully suppress17 the first step of the cascade. Alternatively, molecular isolation enables5-9 studies of pristine systems on surfaces under ultra-high vacuum. If, on the other hand, we want to unleash the reactivity and use π-radical cascades as a synthetic tool, the PLY’s π-delocalized electronic structure offers a unique opportunity to make some positions more reactive than others and thereby enable selectivity control. This principle was demonstrated intermolecularly using steric effects18-20 (Figure 1, middle) and π-extension,21 as well as intramolecularly in oligomeric PLY-based systems.22, 23
To demonstrate that this and related reactions hold a transformative potential for synthesis, we set out to explore if it is possible to utilize the oxidative dimerization of PLY to access curved polycyclic aromatic hydrocarbons. Even though the synthesis of distorted structures requires the build-up of strain,24 we reasoned that such a transformation should be feasible because of the gain of aromatic stabilization energy (Figure 1, dark-gray ring) in the final step of the PLY cascade. Following this idea, we designed biradical 1 (Figure 1, bottom) in which two PLY units (gray fill) are linked via their β-positions using a 2,2′-biphenylene spacer (white fill) to give an eight-membered ring upon their intramolecular ring-fusion (dark-gray fill). Calculations (DFT/M05-2X/6-31G(d,p), Figure S58) revealed that the expected product, picenotetraphenylene (2), possesses a chiral saddle-geometry with a strain energy of ~12 kcal mol−1 (Table S10) and features a twisted peropyrene unit. Here, we demonstrate that this concept works and report25 insights into this π-radical cascade process, as well as the characterization of the final product 2.
The dihydro-precursor of biradical 1, compound 2H-1, was prepared via a convergent synthesis with nine steps in the longest linear sequence (Figure 2). 2,2′-Biphenylene was selected as the linking unit as it provides enough rigidity to achieve the desired selectivity of the σ-dimerization step but leaves enough flexibility for the remaining steps of the cascade to proceed. The 2,2′-biphenylene building block 326 was synthesized by treating 1,2-dibromobenzene with n-butyllithium followed by trimethyl borate (see the SI). The second building block, phenalenone 4, was prepared from naphthalene in six steps, as described27 previously. A twofold Suzuki coupling of 3 with two equivalents of 4 was employed to afford diketone 5 whose structure was validated by X-ray crystallography28 (Figure S25). Subsequently, 5 was reduced with DIBAL−H to give the air-sensitive dihydro-precursor 2H-1 in a 43 % yield over the two steps. Similar to other related systems, 2H-1 was obtained as a mixture of regioisomers, which differ from each other in the location of the methylene groups in the phenalene unit, as observed by NMR spectroscopy (Figure 2) and HRMS (see the commentary in the SI, X-Ray Crystallography section28). The target biradical 1 was generated by oxidation of 2H-1 with p-chloranil and directly used for the subsequent experiments without further purification. All experiments involving 2H-1 and biradical 1 were performed under inert conditions.
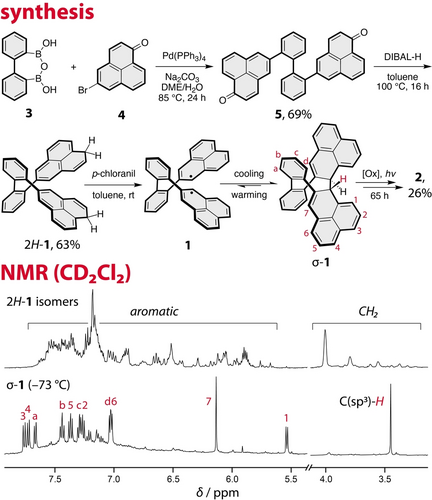
Synthesis and 1H NMR spectrum of precursor 2H-1, and its oxidation with one equivalent of p-chloranil to biradical 1 that is in thermal equilibrium with its σ-dimer (σ-1) observed by 1H NMR spectroscopy at −73 °C.
The transformation of 2H-1 to 2 was monitored by several spectroscopic techniques, including NMR, EPR, and UV/Vis, which allowed the observation of three out of four intermediates of this oxidative cascade. Upon the addition of one equivalent of p-chloranil (the theoretical amount needed for the removal of two hydrogen atoms), the 1H NMR signals that belong to 2H-1 disappear (Figure S12), indicating the formation of biradical species 1. Upon cooling of this mixture, new signals start to appear and at −73 °C, a well-resolved spectrum is obtained (Figure 2). This process is reversible and upon warming, the original spectrum is restored. This behavior supports the expected equilibrium between 1 and its intramolecular σ-dimer (σ-1). Indeed, the assignment of proton and carbon resonances using 2D NMR spectroscopy at −73 °C in CD2Cl2 (see the SI) confirms the structure of σ-1 that is present in the form of a single diastereomer (RR/SS).
The presence of the biradical species 1 was probed by continuous-wave EPR spectroscopy in toluene (c≈10−4 M), revealing a signal with a pronounced hyperfine structure (a triplet of a septet) and an isotropic g value of 2.0025 (Figure 3, top). Interestingly, the experimental proton hyperfine coupling constants (A=−17.8 and 5.1 MHz) do not match the values calculated for the triplet 1 (Figure S45), but those calculated for the partially oxidized monoradical species H-1 (A=−16.6 and 6.8 MHz, DFT/U-M05-2X/EPR-III on M05-2X/6-31G(d,p) geometries, Figure S44). Moreover, the signal is near-identical to that obtained for the reference molecule β-phenyl-PLY, which represents one half of 1 (Figure S28a). The only difference is that the intrinsic linewidth seems to be smaller in the case of the reference molecule, so that the additional smaller proton hyperfine couplings become visible.
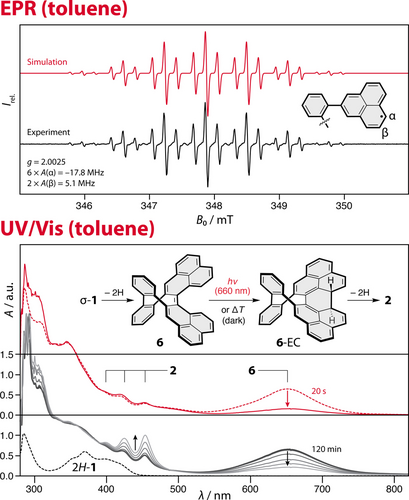
(top) EPR spectrum of a toluene solution of 2H-1 and one equivalent of p-chloranil. (bottom) Observation of intermediate 6 (λmax=654 nm) by UV/Vis spectroscopy and its thermal (black trace) and photochemical (red trace) decay at room temperature in toluene.
These results point toward the presence of either (i) monoradical species H-1 or (ii) biradical species 1 with a negligible spin–spin coupling between the two PLY units. The following experimental observations support the second case: varying the amount of oxidant down to substoichiometric amounts results in (1) an identical EPR signal (Figure S28b) and (2) the appearance of NMR signals that correspond to σ-1 at −73 °C, (3) the EPR signal disappears at temperatures below 230 K and appears again when the temperature is raised (Figure S28c), (4) the NMR signals of σ-1 appear at lower temperatures and broaden at room temperature (Figure S12); (3) and (4) endorse the equilibrium between 1 and σ-1. The DFT calculations further support this scenario and observations. The energy difference between broken-symmetry (BS)-singlet 1 and triplet 1 is <0.01 and 0.12 kcal mol−1 (in favor of the singlet state, U-M05-2X/6-31G(d,p); Tables S4 and S5) for the unfolded and folded conformations, respectively. This indicates a diminished spin–spin interaction, on account of the disrupted conjugation due to orthogonality of the biphenylene linker. The energy of σ-1 is lower by 3.81 kcal mol−1 with respect to the folded BS-singlet 1 (Figure S50).
The next intermediate of the cascade, namely, compound 6, was observed by UV/Vis spectroscopy after the addition of an equivalent amount of p-chloranil to 2H-1 (Figure 3, bottom). A new broad absorption band (λmax=654 nm) appeared instantly and its intensity increased in time until it reached a maximum after 20 min, then it slowly decreased again (black trace). Upon irradiation at 660 nm for 20 s, the band at 654 nm disappears instantly (red trace). These results are consistent with a biphenalenylidene-type intermediate 6, which undergoes both thermal and photochemical electrocyclization to 6-EC (see the SI, page S45) on account of its small HOMO–LUMO gap and diradicaloid character, similar to biphenalenylidene2 and cethrene.29 The TD-DFT calculations for 6 are in agreement with the absorption profile (Tables S6 and S7, Figures S54 and S55) and the energetically favored electrocyclic process (6-EC is lower in energy compared to 6, see Figures S56 and S57). Even though 6-EC could not be observed directly, its intermediacy is supported by the observation that the absorption bands of the final product 2 do not appear instantly after irradiation (Figure 3, bottom, red trace). In contrast, the appearance of these bands is simultaneous with the decrease of the band at 654 nm (6) during the thermal process (black trace). The overall cascade can be performed under both thermal and photochemical reaction conditions, with the latter process being faster. Even though no major side-products were observed, the isolated yield of the final product 2 was 28 % over five consecutive steps (~78 % per step).
The structure of 2 was elucidated by means of 2D NMR spectroscopy (Figures S13–S17) and X-ray diffraction analysis28 (Figure 4, top left), revealing a twisted saddle-shape geometry. The structure of 2 is strained (12.2 kcal mol−1), as estimated using a homodesmotic reaction (Figure S62). The UV/Vis absorption and emission spectra of 2 resemble the features of the spectra obtained previously19 for a flat PP derivative and reveal a Stokes shift of 589 cm−1 (λabs=463 nm, λem=476 nm; Figure 4, top right). The fluorescence quantum yield (ΦF) of 2 is 68 %. With the help of TD-DFT, the lowest-energy absorption band was assigned to the main HOMO→LUMO transition (λ=461.04 nm, f=0.9553; CAM-B3LYP/6-31G(d,p)/PCM on M05-2X/6-31G(d,p) geometry, correction –0.25 eV), with the frontier molecular orbitals resembling those of pristine PP (Figure S59). As a result of its rigid twisted geometry, 2 is chiral and its enantiomers could be resolved using chiral-stationary-phase high-performance liquid chromatography (HPLC; Figure 4, bottom left). The enantiomers displayed mirror-image Cotton effects in their circular dichroism (CD) spectra (Figure 4, bottom right) and their configuration was assigned using TD-DFT calculated transitions (Figure S61). Enantioenriched samples of 2 exhibited circularly polarized luminescence (CPL) in the range of 450–600 nm with a luminescence dissymmetry factor (glum) of 2×10−4, which is comparable to other30 chiral peropyrenes. A high enantiomerization barrier is predicted by DFT (~63 kcal mol−1, M05-2X/6-31G(d,p)), consistent with observation that no racemization occurs at 80 °C (Figure S35). The calculated barrier is in the range of barriers for related benzannulated cyclooctatetraenes suffering from multiple bay-region clashes31 and is on the high end among chiral polycyclic aromatic systems.30, 32
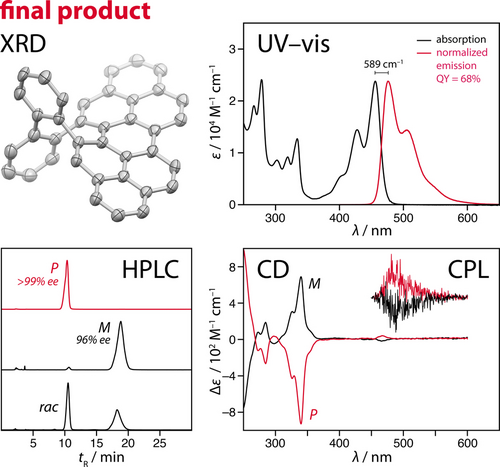
(top left) Solid-state structure of 2 with the thermal ellipsoids shown at 50 % probability level and the hydrogen atoms omitted for clarity. (top right) Absorption and normalized emission spectra of 2 (10−5 M in CH2Cl2) at 298 K. (bottom left) Chiral-stationary-phase HPLC traces of (P)-2, (M)-2, and (±)-2 (Reprosil Chiral-MIA, hexanes/i-PrOH (99 : 1), 1.5 mL/min). (bottom right) CD and CPL spectra of (P)- and (M)-2 (10−5 M in CH2Cl2, λexc=370 nm).
Our study illustrates that we can leverage the typically undesired π-radical reactivity as a method for making novel functional materials—not only by building extended aromatic cores but also via inducing strain to access curved systems. The PLY cascade complements the rather limited pool of methods toward chiral peropyrenes,30, 32-34 while the contortion of the PP unit makes compound 2 a promising singlet-fission material, as highlighted recently for bent pyrenacene35 and rylene diimide36 families. Moreover, by controlling the amount of oxidant, we could showcase a reversible two-state thermal magnetic switch, which is relevant in the context of responsive materials37 for spintronics.
Acknowledgments
This project received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (M.J./716139, INSPIRAL), the Swiss National Science Foundation (SNSF; C.M.C./CRSK-2_190365, M.J./PP00P2_170534, PP00P2_198900 and TMCG-2_213829, CASCADER) and the University of Zurich (UZH; C.M.C./FK-21-131). S.R. acknowledges support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project number 417643975. We would like to thank the S3IT team (www.s3it.uzh.ch, the Service and Support for Science IT team at UZH) for their support and use of the infrastructure, and Prof. Marcel Mayor (University of Basel), to whom this manuscript is dedicated, for his continuous support and fruitful discussions during the PhD committee meetings of A.B. Open Access funding provided by Universität Zürich.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in https://zenodo.org at 10.5281/zenodo.10212222, reference number 10212222.


