Cobalt-Mediated [3+1] Fragmentation of White Phosphorus: Access to Acylcyanophosphanides
Abstract
Despite the accessibility of numerous transition metal polyphosphido complexes through transition-metal-mediated activation of white phosphorus, the targeted functionalization of Pn ligands to obtain functional monophosphorus species remains challenging. In this study, we introduce a new [3+1] fragmentation procedure for cyclo-P4 ligands, leading to the discovery of acylcyanophosphanides and -phosphines. Treatment of the complex [K(18c-6)][(Ar*BIAN)Co(η4-P4)] ([K(18c-6)]3, 18c-6=[18]crown-6, Ar*=2,6-dibenzhydryl-4-isopropylphenyl, BIAN=1,2-bis(arylimino)acenaphthene diimine) with acyl chlorides results in the formation of acylated tetraphosphido complexes [(Ar*BIAN)Co(η4-P4C(O)R)] (R=tBu, Cy, 1-Ad, Ph; 4 a–d). Subsequent reactions of 4 a–d with cyanide salts yield acylated cyanophosphanides [RC(O)PCN]− (9 a–d−) and the cyclo-P3 cobaltate anion [(Ar*BIAN)Co(η3-P3)(CN)]− (8−). Further reactions of 4 a–d with trimethylsilyl cyanide (Me3SiCN) and isocyanides provide insight into a plausible mechanism of this [3+1] fragmentation reaction, as these reagents partially displace the P4C(O)R ligand from the cobalt center. Several potential intermediates of the [3+1] fragmentation were characterized. Additionally, the introduction of a second acyl substituent was achieved by treating [K(18c-6)]9b with CyC(O)Cl, resulting in the first bis(acyl)monocyanophosphine (CyC(O))2PCN (10).
Introduction
Transition-metal-mediated processes offer promising and atom-efficient synthetic routes to organophosphorus compounds derived from white phosphorus (P4), but represent a challenging goal in this field.1 Research over several decades has led to the development of a plethora of early and late transition metal polyphosphido complexes through the activation of P4.2 While coordination chemistry approaches have demonstrated the potential for P4 functionalization, achieving the release of desirable (mono−)phosphorus compounds from the metal center has generally proven difficult. Seminal studies by Peruzzini and co-workers have explored the hydrogenation of P4 using rhodium and iridium hydride complexes.3 More recently, Scheer and co-workers utilized the pentaphosphaferrocene [Cp*Fe(η5-P5)] (Cp*=η5-C5Me5) to prepare asymmetrically substituted phosphines from P4.4 Despite these notable achievements, the successful generation of organophosphorus compounds through transition-metal-mediated P4 functionalization remains limited.
Another highly desirable class of organophosphorus compounds is represented by mono- and bis(acyl)phosphine oxides (MAPOs and BAPOs, Figure 1a). These compounds exhibit intriguing photoactivity, allowing the generation of phosphinoyl and acyl radicals even under weak, visible-light irradiation.5 Recent studies have reported various methods for the synthesis of mono-, bis-, and tris(acyl)phosphines. These methods include reactions of alkali metal phosphanides MPH2 (M=Li, Na, K),6 or phosphaethynolates MPCO7 with electrophiles, as well as the formal insertion of tert-butyl phosphinidene (tBu−P)8 into the C−Cl bond of acyl chlorides. More recently, a one-pot reaction of P4, dilithio reagents, and acyl chlorides has also been explored.9 However, it is important to note that the scope of these methods is often limited, and the resulting products remain bound to the metal center.10 Cummins and co-workers reported the reaction of the terminal phosphide complex [P≡Nb(N[Np]Ar)3]− (A, Np=neopentyl, Ar=3,5-Me2C6H3) with acyl chlorides to give niobacycles of the form B (Figure 1b).11 Subsequent development of this chemistry has seen the release of a P1 moiety through thermolysis, which induces a [2+2] fragmentation, yielding the phosphaalkynes R−C≡P (R=tBu, 1-Ad) and the niobium(V)-oxo product C. This process was reported to form a closed synthetic cycle, as compound A was regenerated through stepwise deoxygenation of C, P4 activation, and reduction. In a separate investigation, the reaction of the trinuclear cyclo-P3 complex anion D with 1-adamantoyl chloride was studied, which yielded the corresponding P3-acylated species E.12 However, E exhibits thermal instability and decomposes above temperatures of −20 °C, limiting further investigation into the reactivity of acyl-substituted Pn ligands. These pioneering works have demonstrated the suitability of P-acylated ligands as precursors for the synthesis of certain monophosphorus compounds. However, to date, only the P1-niobacyle B has been extensively studied in this regard.
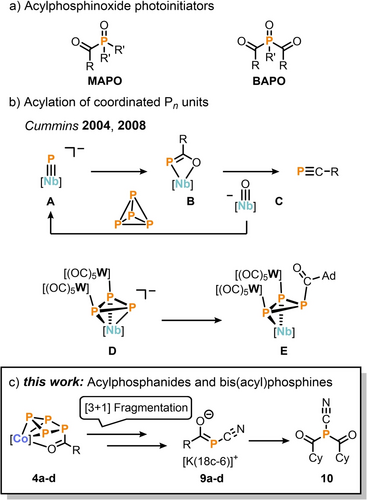
a) Selected industrially applied mono- and bis(acyl)phosphineoxides (MAPOs and BAPOs); R, R′=alkyl or aryl. b) acylation of coordinated Pn-ligands. c) synthesis of acylated mono- and bis(acyl)phosphines starting from P4 by [3+1] fragmentation of acylated tetraphosphido ligands; R=tBu, Cy, 1-Ad, Ph.
Our previous investigation into the reactivity of transition metalate anions with P4 has indicated that anionic polyphosphorus complexes hold potential as versatile tools for the synthesis of unique phosphorus compounds.13 Recently, we reported on the [3+2] fragmentation of pentaphosphido ligands within the coordination sphere of cobalt, leading to new P2 anions [R2PPCN]− (R=Cy, tBu, Ph, N(iPr)2).14 However, compounds containing a PCN unit remain underreported, with a particular scarcity of anionic species, the notable exception being the dicyanophosphide anion [P(CN)2]−.15
Advancing on this strategy, we have synthesized the first acylated cyanophosphanides [RC(O)PCN]− (9 a–d−) through the intermediacy of tetraphosphido complexes [(Ar*BIAN)Co(η4-P4)]− (3−) and [(Ar*BIAN)Co(η3 : η1-P4C(O)R] (4 a–d). The anionic cyclo-P4 complex 3− can initiate P−C bond formation and subsequently undergo [3+1] fragmentation, liberating the acylated P1 unit. Furthermore, the reaction of P1-species [K(18c-6)]9b with acyl chloride yielded bis(acyl)monocyanophosphine (CyC(O))2PCN 10, which possesses the crucial motif found in industrial photoinitiators.5
Results and Discussion
Our study commenced with the preparation of the sterically demanding α-diimine Ar*BIAN (1),16 which was employed as a ligand in our target complexes. Our aim was to suppress the previously reported formation of dinuclear cobalt-P4 complexes by introducing a bulky substituent Ar* on the BIAN ligand, thus facilitating the accessibility of the P42− synthon for functionalization.17 Previous synthetic methods for sterically encumbered BIAN ligands required significant synthetic effort and typically resulted in poor yields.16, 18 However, by templating with ZnCl2, the α-diimine 1 was successfully obtained in a good yield (70 %).19 Subsequently, ligand 1 was treated with the cobaltate [K(thf)0.2][Co(cod)2] (cod=1,5-cyclooctadiene) and 18c-6 in THF to afford [K(18c-6)][(Ar*BIAN)Co(cod)] ([K(18c-6)]2). The complex was isolated as dark brown crystals in good yield (77 %) from a THF/n-hexane mixture. The 1H NMR spectrum of [K(18c-6)]2 (see Figure S3, Supporting Information(SI)) exhibits signals corresponding to Ar*, as well as the characteristic signals of the BIAN backbone at δ=4.21–6.30 ppm.17b, 20
Monitoring via 31P{1H} NMR spectroscopy shows that complex [K(18c-6)]2 reacts quantitatively with white phosphorus to afford the desired mononuclear cyclo-P4 complex [K(18c-6)][(Ar*BIAN)Co(η4-P4)] ([K(18c-6)]3; Scheme 1a). This compound crystallizes as dark purple needles from a toluene/n-hexane mixture. The reaction can be conducted on a multigram scale (>2.7 g), furnishing [K(18c-6)]3 in a good isolated yield of 63 %. This provides an accessible precursor for the subsequent functionalization of the P42− ligand. Single-crystal X-ray diffraction (XRD) analysis (Figure S79, SI) of compound [K(18c-6)]3 revealed a nearly planar cyclo-P4 unit with P−P bond lengths ranging from 2.1539(9) to 2.1772(1) Å (mean: 2.17 Å). These bond lengths lie between typical P−P single and P=P double bond lengths (∑rPP 2.22 Å vs. 2.04 Å),21 indicating the presence of a P42− ligand.13c, 14, 17a, 22 Additionally, the C−C (1.426(3) Å) and C−N (1.335(3) Å and 1.330(3) Å) bond lengths in the ligand backbone of 3 indicate the presence of a radical anionic Ar*BIAN⋅− ligand.23
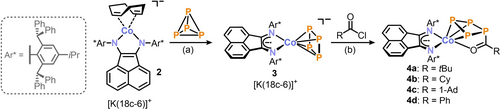
Activation of P4 by [K(18c-6)]2 and subsequent functionalization of the cyclo-P4 unit in [K(18c-6)]3 with acyl chlorides (18c-6=[18]-crown-6, Ar*=2,6-dibenzhydryl-4-isopropylphenyl); reagents/by-products and conditions: a) +P4/−1,5 cyclooctadiene (1,5-cod); THF, r.t., 1 d; b) +RC(O)Cl/−[K(18c-6)]Cl; toluene, r.t., 1 d; yields: [K(18c-6)]2: 77 %, [K(18c-6)]3: 63 %, 4 a: 58 %, 4 b: 54 %, 4 c: 66 %, 4 d: 67 %.
The 31P{1H} NMR spectrum of [K(18c-6)]3 in C6D6 exhibits a sharp singlet at δ=113.0 ppm, which compares well with the value calculated by DFT (δ=137 ppm at the PBE0/def2-TZVP/aug-pcSseg-2 (P) level, see Table S12, SI). In comparison, two other previously reported mononuclear cobalt cyclo-P4 complexes, anionic [(DippPHDI)Co(η4-P4)]− (DippPHDI=bis(2,6-diisopropylphenyl)phenanthrene-9,10-diimine) and neutral [Cp′′′Co(η4-P4)] (Cp′′′=C5H2tBu3), exhibit 31P{1H} NMR resonances at δ=136.5 ppm and δ=175.2 ppm, respectively.14, 22b
To explore the underreported chemistry of acylated polyphosphido ligands, introducing the P-acyl group as a functional group at the tetraphosphido ligand in [K(18c-6)]3 was of particular interest. Treatment of [K(18c-6)]3 with acyl chlorides RC(O)Cl (R=tBu, Cy, 1-Ad, Ph; see Scheme 1(b)) in toluene elicits a color change from purple to magenta.
Crystallization from the reaction mixtures yielded magenta-colored crystals of the acylated tetraphosphido complexes [(Ar*BIAN)Co(η3 : η1-P4C(O)R] (4 a–d) in good yields (54 % to 67 %). Crystallographic studies conducted on three of the complexes, 4 a–c, revealed the presence of an acylated cyclo-P4 ring in a puckered conformation. The cyclo-P4 ring coordinates to Co via three P atoms in an η3 fashion and additionally via the oxygen atom through η1-coordination. Complexes 4 a–c are essentially isostructural. Specifically, in the case of 4 a (see Figure 2a), the P1−P2 (2.2459(9) Å) and P1−P4 (2.2515(6) Å) bond lengths involving the acyl-substituted P atom P1 are slightly longer than expected for typical P−P single bonds (∑rPP 2.22 Å).21 In contrast, the P2−P3 (2.1610(7) Å) and the P3−P4 (2.1547(9) Å) bond lengths are slightly shorter, indicating partial double bond character. The C3−O1 (1.242(3) Å) double bond length falls within the expected range for carbonyl groups (∑rCO 1.24 Å), while the Co1−O1 (2.0741(1) Å) bond length exceeds the sum of the covalent radii for a Co−O single bond (∑rCoO 1.74 Å).21
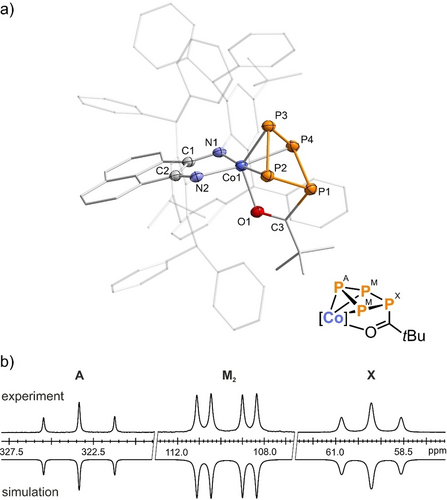
a) Solid-state molecular structure of [(Ar*BIAN)Co(η3 : η1-P4C(O)tBu)] (4 a);45 thermal ellipsoids are shown at the 50 % probability level; hydrogen atoms and disorder are omitted for clarity. Selected bond lengths [Å] and angles [°]: P1−P2 2.2459(9), P2−P3 2.1610(7), P3−P4 2.1547(9), P1−P4 2.2515(6), Co1−P2 2.2974(6), Co1−P3 2.2930(7), Co1−P4 2.2936(8), Co1−O1 2.0741(1), P1−C3 1.889(2), C3−O1 1.242(3), P1−P2−P3 89.83(3), P2−P3−P4 87.25(3), P4−P1−P2 82.92(2); b) experimental (upward) and simulated (downward) 31P{1H} NMR spectra of 4 a, with nuclei assigned to an AM2X spin system: δ(PA)=323.3 ppm, δ(PM)=109.7 ppm, δ(PX)=59.2 ppm, 1JAM=−342 Hz, 1JMX=−106 Hz, 2JAX=7 Hz. The spectra of the related compounds 4 b–d are very similar (see SI); [Co]=(Ar*BIAN)Co.
Each of the complexes 4 a–d features an AM2X spin system in the 31P{1H} NMR spectra (see Figure 2b for 4 a; see Supporting Information for similar spectra of 4 b–d). The resonances of 4 a (δ=323.3 (PA), 109.7 (PM), 59.2 (PX) ppm; c.f. the DFT-calculated chemical shifts of 315 (PA), 99 (PM) and 67 ppm (PX))—especially PA, the coordinating phosphorus nucleus—are deshielded in comparison to related neutral cobalt complexes and niobacycles B.11, 24
Quantum chemical calculations performed at the BP86/def2-TZVP level of theory predict the C=O stretching vibration for 4 a at ṽCO=1462 cm−1 (see Figure S97, SI), which is between the regions characteristic of a C=O double and single bond (1700 cm−1 vs. 1100 cm−1).25 However, in the ATR-IR spectrum, the C=O vibration overlaps with BIAN C−N vibrations in the fingerprint region, making unambiguous identification challenging. Similar behavior was reported for niobacycles B.11
Having demonstrated that the P42− ligand of anion 3− was readily functionalized to give 4 a–d, our focus shifted toward isolating new organophosphorus compounds by displacing the phosphorus moiety from the coordination sphere of the cobalt center. To achieve this, 4 a–d were reacted with neutral cyanide Me3SiCN (Scheme 2a). Specifically, the addition of one equivalent of substrate to a solution of 4 a (R=tBu) resulted in a color change from magenta to dark green. Analysis of the 31P{1H} NMR spectrum of the reaction mixture revealed the complete consumption of 4 a, with the formation of a new species 5 a, exhibiting four distinct resonances in a 1 : 1 : 1 : 1 ratio (vide infra). Equivalent reactions of 4 b–d toward Me3SiCN gave very similar 31P{1H} NMR spectra, indicating the formation of compounds analogous to 5 a (Figure S47, SI).
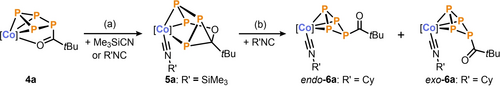
Rearrangement and partial release of phosphoracylic ligands in 4 a is induced by trimethylsilyl cyanide (Me3SiCN) or isocyanides R′NC (R′=Cy, tBu, Mes, Ph); reagents and conditions: a) +1.3 eq. Me3SiCN; toluene, r.t., 14 h; b) +10.0 eq. CyNC; toluene, r.t., 2 h; yields: 5 a: 77 %, 6 a: 57 %; [Co]=(Ar*BIAN)Co; for further combinations see also Table S8 and Figures S46-52 in the SI.
Compound 5 a was isolated as a green crystalline solid in 75 % yield after crystallization from toluene/n-hexane at low temperature (−35 °C).26 Analysis of 5 a by XRD revealed an edge-bridged trigonal prismane derivative resulting from the insertion of the acyl group into one of the P−P bonds of 4 a (Figure 3a).27 The prismane core consists of two triangular planes—one formed by cobalt and two phosphorus atoms, and the other by the carbonyl carbon and two phosphorus atoms. The P4−C4 edge is bridged by the carbonyl oxygen atom. Thus, the isocyanide substrate has displaced the coordination of the carbonyl to the cobalt center in 4 a, leading to rearrangement of the P4C(O)R ligand. While related compounds containing prismatic units based on catena-E4 (E=P, As) moieties are typically stabilized by two metal fragments, 5 a represents an unusual example where the P4 core is supported by only one metal fragment and substituted with an organic residue.28 The Co1−P3−P4 plane is nearly parallel to the P1−P2−C4 plane, with a twist angle of 14.2°. The P1−P2 and P2−P3 bond lengths (2.1961(1) and 2.207(1) Å, respectively) fall within the range of P−P single bonds (∑rPP 2.22 Å), while the shorter P3−P4 bond (2.1355(1) Å) implies the retention of significant double bond character.21 Similar discrepancies between P−P bond lengths have been observed in previous prismane-derived complexes.28b, 28d, 28e This suggests that bonding of the polyphosphorus ligand in 5 a is best described as a localized Co1−P1 σ-bond, with the P3−P4 unit engaging in π-coordination to the cobalt center. While the cyanide Me3SiCN was used as the reactant, the crystal structure for 5 a reveals the coordination of the corresponding isocyanide, Me3SiNC. It is known that an equilibrium exists between the cyanide and isocyanide isomers of Me3SiCN.29 Thus, the coordination of Me3SiCN to the cobalt center induces a quantitative isomerization, favoring the coordination of a silyl isocyanide (−C≡NSiMe3) ligand over the cyanide (−N≡CSiMe3) ligand due to energetic considerations. This is supported by a sharp vibration mode at ṽCN=2012 cm−1 in the infrared spectrum and a broadening of the C≡N 13C{1H} NMR resonance at δ=195.0 ppm (Δν1/2=25 Hz), corroborating the coordination of the carbon to the cobalt center in 5 a.25
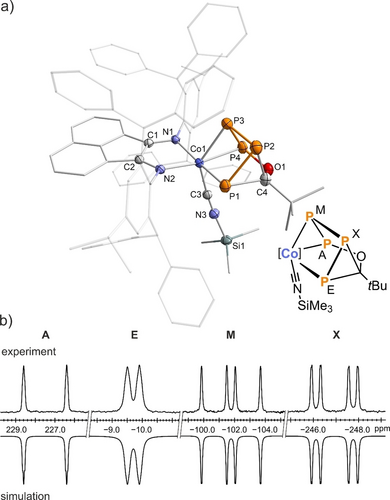
a) Solid-state molecular structure of [(Ar*BIAN)Co(Me3SiNC)(η2 : η1-P4COtBu)] (5 a);45 thermal ellipsoids are shown at the 50 % probability level; hydrogen atoms and disorder are omitted for clarity. Selected bond lengths [Å] and angles [°]: P1−P2 2.1961(1), P2−P3 2.207(1), P3−P4 2.1355(1), Co1−P1 2.2895(7), Co1−P3 2.3330(8), Co1−P4 2.2987(8), C4−O1 1.432(3), P4−O1 1.6722(2), Co1−C3 1.851(2), Co1−C3−N3 174.3(2), C3−N3−Si1 176.14(2); b) experimental (upward) and simulated (downward) 31P{1H} NMR spectra of 5 a with nuclei assigned to an AEMX spin system: δ(PA)=228.1 ppm, δ(PE)=−10.7 ppm, δ(PM)=−102.4 ppm, δ(PX)=−245.1 ppm, 1JAM=−355 Hz, 1JMX=−267 Hz, 1JEX=−64 Hz, 2JAX=8 Hz, 2JME=9 Hz, 3JAE=−11 Hz. The spectra of the related compounds 5 b–r are very similar (see SI, Figures S47–S52); [Co]=(Ar*BIAN)Co.
The 31P{1H} NMR spectrum of 5 a in C6D6 exhibits four resonances corresponding to an AEMX spin system. These appear as two doublets (δ=228.2 (PA) ppm and δ=−10.7 (PE) ppm) and two doublets of doublets (δ=−102.5 (PM) ppm and δ=−245.2 (PX) ppm) (Figure 3b), characteristic of an asymmetric catena-P4 unit.13d, 30 The 31P{1H} NMR spectrum was successfully simulated by an iterative fitting procedure (Figure S29, SI), which identified small 2JPP and 3JPP couplings. The 1JPP coupling constants vary widely from −355 to −64 Hz. The resonance attributed to P1 at δ=−10.7 ppm is significantly broadened (Δν1/2=99 Hz; 1JPP=−64 Hz), likely due to interactions with the quadrupolar 59Co nucleus, which is consistent with the Co−P1 bond constituting the major cobalt-phosphorus interaction.31
Considering the observed isomerization to the isocyanide for neutral cyanide,29 we proceeded to react compounds 4 a–d with alkyl and aryl isocyanides R′NC (R′=Cy, tBu, Mes, Ph) (Scheme 2b). Initially, the formation of analogues of the previously described complex, 5 a were also observed in these reactions (Figure S48, SI). Continuous addition of up to 10 equivalents of isocyanide leads to a clean reaction and full conversion to two isomeric η3-cyclo-P4 complexes endo-6 and exo-6. These stereoisomers only differ by the position of the acyl substituent. A similar mixture of isomers was observed in reactions with related CoPn complexes.30c, 32 The transformation of 5 to 6 could also be induced by heat, albeit with concomitant decomposition of 5.
A wide range of reactions of 4 a–d toward different isocyanides and isoelectronic carbon monoxide have been explored, which gave very similar results. Further details of the reactions and the resulting complexes 6 b–p, observed by 31P{1H} NMR spectroscopy, can be found in the Supporting Information (Table S8, Figures S46–52).
Specifically, in the reaction of 4 a (R=tBu) toward cyclohexyl isocyanide both exo- and endo-isomers of 6 a are formed at low temperature, as evidenced by a variable temperature (VT) NMR monitoring experiment (Figure S96, SI). Additional DFT calculations revealed that endo-6 a and exo-6 a are isoenergetic (see the Supporting Information for details).
Both stereoisomers of 6 a co-crystallize from a saturated n-hexane solution in 57 % overall yield as dark green crystals, which were analyzed by XRD. The molecular structures of 6 a are analogous to 4 a, with the P4C(O)R ligand coordinated to the cobalt center in an η3 fashion (Figure 4), while the carbonyl moieties have been displaced from coordination to the cobalt by one molecule of isocyanide. The bond lengths of the η3-P4C(O)R ligand in the solid-state molecular structures of 6 a closely agree with those of the η3 : η1-P4C(O)R ligand in 4 a–c.
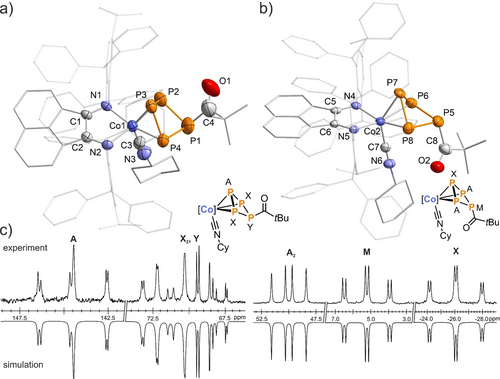
Solid-state molecular structures of a) endo-[(Ar*BIAN)Co(CyNC)(η3-P4C(O)tBu)] (endo-6 a) and b) exo-[(Ar*BIAN)Co(CyNC)(η3-P4C(O)tBu)] (exo-6 a) in the co-crystal;45 thermal ellipsoids are shown at the 50 % probability level; hydrogen atoms, disorder and non-coordinating solvent molecules are omitted for clarity. Selected bond lengths [Å] and angles [°] of endo-6 a: P1−P2 2.218(3), P1−P4 2.232(3), P2−P3 2.191(2), P3−P4 2.165(3), Co1−P2 2.3000(2), Co1−P3 2.2983(2), Co1−P4 2.3027(2), Co1−C3−N3 178.4(5); c) experimental (upward) and simulated (downward) 31P{1H} NMR spectra of endo-6 a with nuclei assigned to an AX2Y spin system: δ(PA)=144.5 ppm, δ(Px)=71.7 ppm, δ(PY)=68.6 ppm, 1JAX=−318 Hz, 1JXY=−165 Hz, 2JAY=−5 Hz; exo-6 a: δ(PA)=50.0 ppm, δ(PM)=5.2 ppm, δ(PY)=−26.1 ppm, 1JAX=−308 Hz, 1JAM=−207 Hz, 2JMX=29 Hz.
In contrast, the 31P{1H} NMR signals of endo-6 a and exo-6 a differ markedly from each other and from those of 4 a–d (Figure 4c). Endo-6 a features an AX2Y spin system in C6D6, in which the signal for the coordinating phosphorus atom P3 is shifted significantly upfield in comparison to 4 a (δ=143.3 ppm for endo-6 a versus δ=323.3 ppm for 4 a). In comparison to endo-6 a, the resonances constituting the A2MX spin system observed for exo-6 a are shifted further upfield. The considerable differences in the chemical shifts of the endo and exo isomers of 6 a are nicely reproduced by our DFT calculations and correlate well with the experimental values (Table S11, SI). The different orientations of the −C(O)R substituents in solution, leading to reduced orbital overlap of the phosphorus atoms, are also evident in the 31P{1H} NMR spectra, with greater 1JPP coupling constants observed for the exo-isomer in 6 a (exo-6 a : 1JAM=−207 Hz vs. endo-6 a: 1JXY=−165 Hz). The full set of parameters, including simulation by an iterative fitting procedure, can be found in the Supporting Information (Figure S35–36). In the ATR-IR spectrum of 6 a, the bands at ṽCO=1599 and 1640 cm−1, respectively, can be attributed to the C=O stretching vibration.25
Additional single-crystal XRD data was obtained for endo-6 a, where only one isomer was observed in solid state, as well as further combinations of R and R′ in exo-6 d (R=Ph, R′=Cy) and endo-6 e (R=tBu, R′=tBu; Figures S85–87, SI). During XRD analysis of 6 a, crystals of a minor side product, [(Ar*BIAN)Co(CyNC)2(η1-P4COtBu)] (7), were also discovered. Structural analysis of these revealed a cobalt complex bearing two isocyanide ligands. This saturation of the coordination sphere is facilitated by the severance of most of the cobalt-phosphorus interactions, resulting in an η1-coordinated [1.1.0]bicyclotetraphosphane-1,4-diyl (“P4 butterfly”) ligand.33 A more detailed discussion of 7 can be found in the Supporting Information (Figure S88).
To gain further insight into the distribution of isomers, a VT NMR spectroscopic analysis of the isolated crystalline material of 6 a was conducted. The crystals were dissolved in toluene-d8 at −80 °C and the temperature was gradually increased while monitoring by 31P{1H} NMR spectroscopy (Figure S94-95, SI). The spectra at low temperature show predominantly the signals assigned to exo-6 a. An increase in temperature leads to an increase in signal intensity for endo-6 a. This observation was attributed to crystal packing effects, which likely influence the solid-state structures and lead to a preference for the crystallization of one isomer over the other. Once the isomerization has occurred and an equilibrium established, cooling the solution back down to low temperature did not reassert a single isomer as a significantly major species in the mixture.
To investigate whether the stronger cyanide anion, CN−, of certain cyanide salts would completely cleave an organophosphorus fragment from the complexes, 4 a–d were reacted with two equivalents of [M]CN ([M]=nBu4N+, Et4N+, K(18c-6)+).14, 34 This resulted in the selective formation of cyclo-P3 cobalt complex [(Ar*BIAN)Co(CN)(η3-P3)]− (8−) and the acylated cyanophosphanides [RC(O)PCN]− (9 a–d−) (Scheme 3). This was initially indicated by the 31P{1H} NMR spectra, in which two singlets were observed in a 3 : 1 integral ratio (Figures S53–56, SI).
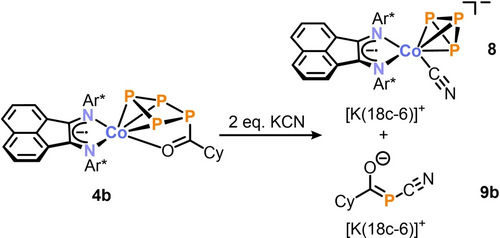
Cyanide induced [3+1] fragmentation of 4 b; reagents/by-products and conditions: +2.2 eq. KCN/+2.2 eq. 18c-6; THF, r.t., 3 d; yields: [K(18c-6)]8: 60 %, [K(18c-6)]9b: 31 %.
The observed chemical shift of δ=−218.7 ppm for [K(18-6)]8 is close to the reported values for [(DippPHDI)Co(η3-P3)(CN)]− (δ=−193.2 ppm), [{B10H10C2(P3Mes2)}Co(η3-P3)]− (δ=−250.9 ppm, Mes=2,4,6-Me3C6H2), and other related cyclo-P3 complexes.14, 24, 35
Acylcyanophosphanides, to our knowledge, have not been reported previously.36 The 31P{1H} NMR signals of 9 a–d− (9 a−: δ=−44.0 ppm, 9 b−: δ=−45.2 ppm, 9 c−: δ=−45.5 ppm, 9 d−: δ=−30.1 ppm) are noticeably shifted upfield compared to the 31P{1H} NMR resonance of the related anion [PhPCN]− (δ=70.3 ppm).37 The reaction of 4 a–d with the CN− anion represents a remarkable [3+1] fragmentation of a tetraphosphido ligand to yield a cyclo-P3− species and an organic monophosphorus compound. While a few transition-metal-mediated [3+1] fragmentations of P4 are known in which the generated P3 and P1 moieties remain coordinated to a transition metal atom,28b, 35b, 38 the release of P1 species from polyphosphorus ligands has rarely been observed.3, 4, 33f, 35a, 39
In the case of R=Cy, the products [K(18c-6)]8 and [K(18c-6)]9b are easily separated by fractional crystallization. [K(18c-6)]8 crystallizes from the concentrated toluene reaction mixture at room temperature, affording purple crystals in 60 % yield. XRD analysis confirmed the coordination of the cyclo-P3 and cyanide ligand to the cobalt center (Figure 5a). The Co−C (1.931(9) Å) and C−N (1.158(4) Å) bond lengths, as well as the CN stretching vibration (ṽCN=2069 cm−1), fall within the typical range for cobalt cyanide complexes.25, 40, 41 The cyclo-P3 ring coordinates to the metal center in a η3 fashion, with average P−P (2.143(7) Å) and Co−P (2.302(9) Å) distances comparable to reported anionic cobalt cyclo-P3 complexes.14, 24, 35 Colorless crystals of [K(18c-6)]9 b were isolated in 31 % yield from the mother liquor at −35 °C. Due to similar solubility, further fractions of isolated crystalline material of [K(18c-6)]9 b contained also small amounts of [K(18c-6)]8. Figure 5b displays the solid-state molecular structure of [K(18c-6)]9b, which features a P1 anion with acyl- and cyanide substituents. Both the oxygen and the nitrogen atoms coordinate to the potassium counterion. Additional single-crystal XRD data was obtained for isostructural [K(18c-6)]9 a and [K(18c-6)]9 d and is given in the Supporting Information (Figures S91–92).
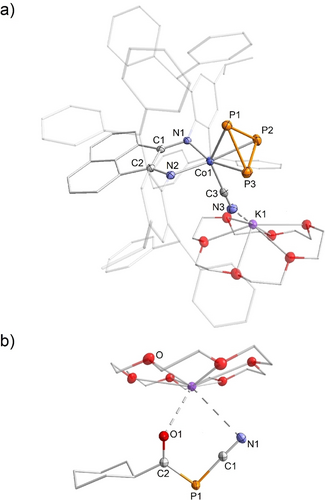
Solid-state molecular structures of a) [K(18c-6)][(Ar*BIAN)Co(CN)(η3-P3)] ([K(18c-6)]8) and b) K(18c-6)][CyC(O)PCN] ([K(18c-6)]9b);45 thermal ellipsoids are shown at the 50 % probability level; hydrogen atoms and disorder are omitted for clarity. Selected bond lengths [Å] and angles [°] of [K(18c-6)]8: P1−P2 2.1318(5), P1−P3 2.1306(5), P2−P3 2.1682(4), Co1−P1 2.3070(4), Co1−P2 2.3001(4), Co1−P3 2.3014(3), Co1−C3 1.9323(1), C1−N1 1.3211(2), C3−N3 1.1583(2), C1−C2 1.4486(2), K1−N3 2.9111(1), P1−P2−P3 59.396(2), P1−P3−P2 59.452(2), P2−P1−P3 61.152(2), Co1−C3−N3 177.83(1); [K(18c-6)]9b: P1−C1 1.7789(1), P1−C2 1.7960(1), C1−N1 1.1519(2), C2−O1 1.2397(1), P1−C1−N1 176.77(9), C1−P1−C2 95.08(5).
Specifically in the case of 9 b−, the similar P1−C1 (1.7789(1) Å) and P1−C2 (1.7960(1) Å) bond lengths lie between those expected for a P=C double and a P−C single bond (∑rPC 1.69 Å vs. 1.86 Å), indicating partial delocalization.21 Furthermore, the C1−N1 (1.1519(2) Å) bond length of the nearly linear PCN group (P1−C1−N1 176.8(8)°) is comparable to that of the cyanophosphanide [Na(18c-6)][P(SiPh3)(CN)] (C−N 1.161 Å, P−C 1.761 Å) reported by Grützmacher and co-workers.42 On the other hand, the C−N (1.248(5) Å) bond length of 1-aza-phospha-allenide [iPr=N=C=P]−is noticeably longer than that of 9 b−, and the P−C (1.603(3) Å) bond length is shorter.
These observations suggest several contributing resonance structures analogous to those proposed for [P(SiPh3)(CN)]−.42, 43 A natural resonance theory analysis conducted at the TPSS/def2-TZVP level of theory revealed that the phosphaenolate resonance form I (37.4 %) is the primary contributor to the electronic ground state, contrasting with the contributions of 1-aza-3-phosphaallenide II and phosphide III (Scheme 4). In comparison, calculations for the related compound [P(SiPh3)(CN)]− showed a significantly higher contribution to the phosphide form (76.4 %).43
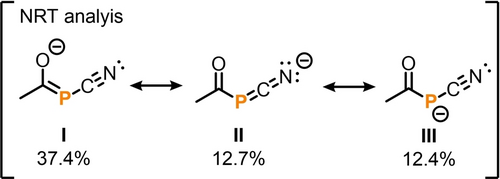
Natural resonance theory (NRT) weights for the dominant Lewis structures of the model cyanophosphanide 9-Me− (R=Me) (TPSS/def2-TZVP) are provided. Comparable ratios for 9 b− can be found in the SI.44
The IR spectrum of 9 b exhibits two characteristic stretching vibrations at ṽCN=2101 cm−1 and ṽCO=1544 cm−1, which are in good agreement with the calculated values (ṽCN=2099 cm−1 and ṽCO=1565 cm−1), as well as with those of other alkyl cyanophosphanides [RP(CN)]− (R=Me, Et, Ph; ṽCN=2080 to 2160 cm−1).37 These values are higher than that reported for cyanodiphosphanide [tBu2PPCN]− (ṽCN=2049 cm−1).14 In contrast, the CO stretching frequency is lower than expected for typical organic compounds (≈1700 cm−1), indicating the relatively high contribution of Lewis type formula I (Scheme 4).25
The [3+1] fragmentation reaction mechanism of 4 a–d by M[CN] is proposed to involve an initial attack of a cyanide anion at the cobalt center, displacing the coordination of the carbonyl and forming an anionic species analogous in structure to neutral 6 (vide supra, also see the SI, Scheme S1). Subsequent nucleophilic attack by a second cyanide anion at the acyl-substituted P atom leads to the release of 9 a–d− and the formation of the cyclo-P3 species 8−. The reaction rate is influenced by the steric demands of the substituents in 4 a–d and even more by the solubility of the cyanide source [M]CN. Monitoring the reaction between 4 d (R=Ph) with [Et4N]CN over a 14-hour period using 31P NMR spectroscopy revealed several intermediate sets of signals that closely resemble an AX2Y and an A2MX spin system, exhibiting similar chemical shifts as observed for endo- and exo-6 a (Figure S93, SI). Unfortunately, the formation of these intermediates in only minor quantities has impeded the successful isolation and characterization of them so far. Nevertheless, the 31P{1H} NMR spectroscopic data indicate that analogues of the previously described complex, 6, featuring exo- and endo-η3-P4C(O)R ligands may serve as potential intermediates in the reaction.
A second acyl substituent can be introduced to 9 b− to generate a bis(acyl)monocyanophosphine (Scheme 5). Therefore, performing salt metathesis of [K(18c-6)]9b with CyC(O)Cl yields (CyC(O))2PCN (10), which was isolated as a colorless oil in 81 % yield. The 31P{1H} NMR spectrum of 10 exhibits a sharp singlet at δ=−8.2 ppm, which is shifted slightly upfield compared to the alkyl-substituted bis(acyl)phosphine P(C(O)Ad)(C(O)Ph)tBu (δ=37 ppm).8 In the 13C{1H} NMR spectrum, two characteristic doublet resonances can be assigned to the acyl (δ=211.2 ppm; 1JPC=52 Hz) and cyano carbon atoms (δ=117.1 ppm; 1JPC=62 Hz). In comparison, the resonances for [K(18c–6)]9b were observed at δ=238.1 ppm for the acyl and δ=136.7 ppm for the cyano carbon atoms.
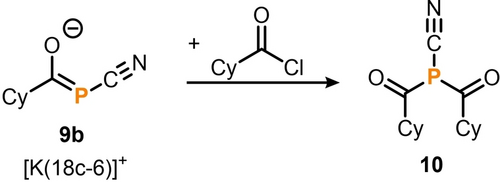
Functionalization of [K(18c-6)]9b with cyclohexanecarboxylic acid chloride to 10; reagents/by-products and conditions: +CyC(O)Cl/−[K(18c-6)]Cl; C6D6, r.t., 1 h; isolated yield: 81 %.
Additionally, the IR spectrum of 10 displays CO stretching frequencies at ṽCO=1715 and 1681 cm−1, which agree well with the calculated values (ṽCO=1709 and 1693 cm−1), confirming the constitution of the bis(acyl)cyanophosphine (CyC(O))2PCN.
Conclusion
In this study, we have synthesized the acylated tetraphosphido complexes [(Ar*BIAN)Co(η3 : η1-P4COR)] (4 a–d) with various alkyl and aryl substituents using a two-step process involving P4, [K(18c-6)]2 and RC(O)Cl. These ligands provide a platform for the study of P-acylated ligands. Treatment of the P4C(O)R complexes with trimethylsilyl cyanide and isocyanides resulted in P−Co bond cleavage, leading to the formation of pnictogen derivatives, including prismane in 5, as well as endo- and exo-isomers of η3-coordinating tetraphosphido ligands (6). Additionally, treatment of 4 a–d with two equivalents of the cyanide anion facilitated the release of acylcyanophosphanides RC(O)PCN− 9 a–d− through a remarkable [3+1] fragmentation process, resulting in the formation of a cyclotriphosphido cobalt complex 8−. Monitoring of the [3+1] fragmentation reaction provided insight into the involvement of intermediates similar to 6, which have rearranged polyphosphorus ligands and are considered key intermediates en route to the anions 8− and (9 a–d−). Additionally, we have synthesized the bis(acyl)cyanophosphine (CyC(O))2PCN (10), highlighting the useful reactivity of these anions. Overall, our findings demonstrate the potential of metalate activation and functionalization of P4 in accessing new (poly-)phosphorus species. We anticipate that this approach will open up avenues for the synthesis of unique phosphorus compounds in future research endeavors. Ongoing investigations are focused on further exploring these possibilities.
Acknowledgments
We thank Daniel Schmidhuber for assistance with quantum chemical calculations, Verena Streitferdt for assistance with NMR experiments and the anonymous referees for their insightful comments on this work. Generous financial support of this work by the European Research Council (ERC, CoG 772299) and the Deutsche Forschungsgemeinschaft (DFG, project WE4621/3-2 and WO1496/7-2) is gratefully acknowledged. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




