Acyl and CO Ligands in the [Fe]-Hydrogenase Cofactor Scramble upon Photolysis
Abstract
[Fe]-hydrogenase harbors the iron-guanylylpyridinol (FeGP) cofactor, in which the Fe(II) complex contains acyl-carbon, pyridinol-nitrogen, cysteine-thiolate and two CO as ligands. Irradiation with UV-A/blue light decomposes the FeGP cofactor to a 6-carboxymethyl-4-guanylyl-2-pyridone (GP) and other components. Previous in vitro biosynthesis experiments indicated that the acyl- and CO-ligands in the FeGP cofactor can scramble, but whether scrambling occurred during biosynthesis or photolysis was unclear. Here, we demonstrate that the [18O1-carboxy]-group of GP is incorporated into the FeGP cofactor by in vitro biosynthesis. MS/MS analysis of the 18O-labeled FeGP cofactor revealed that the produced [18O1]-acyl group is not exchanged with a CO ligand of the cofactor, indicating that the acyl and CO ligands are scrambled during photolysis rather than biosynthesis, which ruled out any biosynthesis mechanisms allowing acyl/CO ligands scrambling. Time-resolved infrared spectroscopy indicated that an acyl-Fe(CO)3 intermediate is formed during photolysis, in which scrambling of the CO and acyl ligands can occur. This finding also suggests that the light-excited FeGP cofactor has a higher affinity for external CO. These results contribute to our understanding of the biosynthesis and photosensitive properties of this unique H2-activating natural complex.
In the hydrogenotrophic methanogenic pathway, H2-forming methylene-tetrahydromethanopterin (methylene-H4MPT) dehydrogenase (Hmd or [Fe]-hydrogenase) catalyzes the reversible hydride transfer from H2 to methenyl-H4MPT+.1 [Fe]-hydrogenase contains the iron-guanylylpyridinol (FeGP) cofactor as the prosthetic group, in which a mono-nuclear low spin Fe(II) is complexed with the 6-acylmethyl substituent of the pyridinol, the pyridinol nitrogen, two CO ligands, and a cysteine thiolate (Figure 1a).2 The cysteine-thiolate anchors the cofactor to the protein.2d, 3 CO ligands are also observed in the dinuclear metal complexes of [NiFe]- and [FeFe]-hydrogenases;4 however, the acyl ligand is unique to [Fe]-hydrogenase. In the enzyme-bound form, the iron coordination site trans to the acyl ligand is occupied by a water molecule and is proposed as the H2 binding site in the catalytic reaction in the activated form of the enzyme.1b, 2g
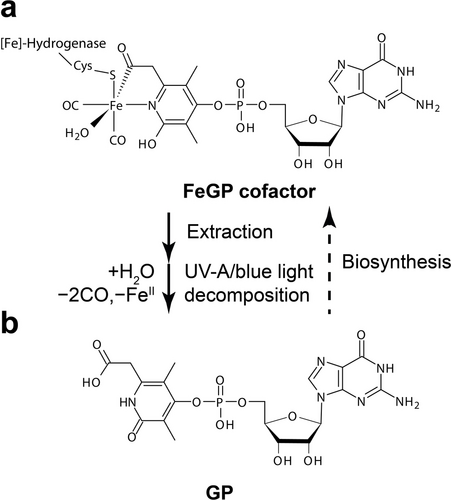
Structure of the FeGP cofactor and the photolysis product. (a) The FeGP cofactor bound to [Fe]-hydrogenase. (b) The light decomposition product 6-carboxymethyl-3,5-dimethyl-4-guanylyl-2-pyridone (GP). GP is used as a biosynthetic precursor of the FeGP cofactor.
The FeGP cofactor can be extracted by denaturing [Fe]-hydrogenase with methanol in the presence of a stabilizing reagent, such as 2-mercaptoethanol or dithiothreitol (DTT), under alkaline conditions.5 The FeGP cofactor can also be extracted by acetic acid.5b The extracted FeGP cofactor is stabilized by the binding of external ligands provided as the stabilization reagents.2d, 2e, 5b An excess of stabilization reagents is needed in the solution to stabilize the FeGP cofactor,6 which indicates that the reagents only weakly bind to the cofactor. By mixing the extracted FeGP cofactor with the [Fe]-hydrogenase apoenzyme, the FeGP cofactor binds to the protein and the fully active [Fe]-hydrogenase holoenzyme is constituted. Upon generation of the holoenzyme, the external ligands are exchanged with a cysteine-thiolate of the protein and a water molecule.3 Accordingly, semisynthetic [Fe]-hydrogenase was constructed by mixing mimic complexes and the apoenzyme.7
Irradiation with UV-A/blue light decomposes the FeGP cofactor in both the enzyme-bound and extracted forms.9 Infrared spectroscopic analysis indicated that the CO ligands are dissociated from the FeGP cofactor by light irradiation.2b By photolysis, the acyl ligand of the isolated FeGP cofactor is hydrolyzed to form the 6-carboxymethyl group of 6-carboxymethyl-3,5-dimethyl-4-guanylyl-2-pyridone (GP) (Figure 1b).2a, 5b GP is also known as a precursor of biosynthesis of the FeGP cofactor8, 10
The FeGP cofactor is biosynthesized by the reactions catalyzed by HcgA−G proteins. GP is produced by the reactions by HcgA, HcgB and HcgC.10, 11 The CO ligand is biosynthesized from CO gas or an unknown organic substance by HcgG.8, 10 The carboxy group of GP is proposed to be converted to the acyl ligand by the consecutive biosynthetic reactions of HcgE, HcgF and HcgG.8, 10, 11c, 12 The hypothesis of acyl-ligand biosynthesis was supported by the observation that 6-decarboxylated GP did not produce active [Fe]-hydrogenase in in vitro biosynthesis and up to two 13C were incorporated into the FeGP cofactor from [13C]-CO (Figure 2a).8 However, in contrast, when the [13C]-CO enriched FeGP cofactor was decomposed by light, GP produced by photolysis contained substantial 13C at the carboxy group, which is produced by hydrolysis of the acyl ligand.8 This can be explained as follows: (i) a part of the acyl ligand is biosynthesized from external CO, and/or (ii) the acyl and CO ligands were scrambled during biosynthesis and/or photolysis.8, 13
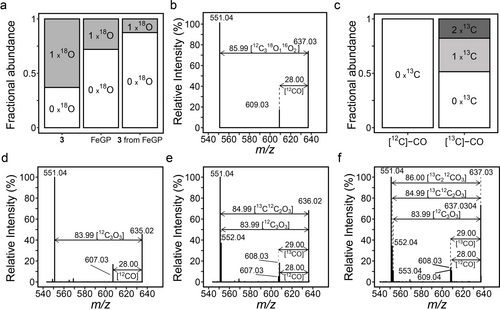
MS analysis of the stable isotope-labeled FeGP cofactor and GP. (a) The fractional abundance of the 18O0- and 18O1-isotopologues of GP produced by photolysis of the FeGP cofactor in [18O]-H2O (GP), the FeGP cofactor synthesized by in vitro biosynthesis from [18O1]-GP (FeGP) and the photolysis product GP from the [18O1]-FeGP cofactor (GP from FeGP). (b) MS/MS of the 18O1-isotopologues of the [18O1]-FeGP cofactor. (c) 13C isotope enrichment of the FeGP cofactor synthesized in in vitro biosynthesis under [13C]-CO was indicated by the fractional abundance of the corresponding 13C isotope isomers. This panel is reproduced from Schaupp et al.8 (d-f) MS/MS spectra of 13C0 (d), 13C1 (e) and 13C2 (f) isotopologues of the [13C]-CO-labeled FeGP cofactor
[Fe]-hydrogenase is inhibited by external CO, in which CO is bound at the FeGP cofactor in the enzyme, as revealed by infrared spectroscopy.2b, 9 The external CO ligand was not observed to scramble in the inhibited enzyme2b although it is known that the CO ligands of mimic complexes of [FeFe]-hydrogenase scramble.14 For the isolated FeGP cofactor, the CO ligand can only bind at low temperatures (−75 °C), but no ligand scrambling was reported.2c Thus, the exchange reaction of the acyl and CO ligands of the FeGP cofactor is not well understood; however, it is known that the CO and acyl ligands of mimic complexes of the FeGP cofactor can be exchanged with external [13C]-CO,15 and UV-A light accelerated the rate of CO incorporation and scrambling of a mimic complex.15b Studies on ligand scrambling are beneficial because they can provide insights into the catalytic properties of the complexes.16
In this communication, we confirmed that the acyl ligand is biosynthesized from the carboxy group of GP using in vitro biosynthesis with [18O1-carboxy]-labeled GP. MS/MS analysis of the labeled FeGP cofactors indicated that the CO and acyl ligands scramble only during photolysis rather than biosynthesis. The time-resolved infrared spectroscopy data of the FeGP cofactor indicated the presence of an acyl-Fe(CO)3 intermediate during photolysis. Based on the findings, we propose a photolytic mechanism that explains the scrambling of the CO and acyl ligands during photolysis.
Mass spectrometry (MS) was used to confirm that the carboxy group of GP was converted to the acyl ligand of the FeGP cofactor as follows. First, we checked the incorporation of GP into the FeGP cofactor using [15N]-labelling experiments (Supplementary result and Figure S1). Next, the [18O1-6-carboxy]-GP was prepared by hydrolyzing the FeGP cofactor by photolysis in [18O]-H2O,5b after which 63 % of GP contained one 18O (Figure 2a). The FeGP cofactor was produced by in vitro biosynthesis using [18O1-carboxy]-GP as the precursor. MS analysis of the resulting 18O-enriched FeGP cofactor indicated that only one 18O is incorporated into the cofactor with an occupancy of ≈28 %. The decrease in 18O occupancy from GP to the FeGP cofactor is consistent with the predicted reaction, in which only one of two carboxy oxygen atoms is converted to the acyl ligand (Figure 2a). Photolysis of the 18O-enriched FeGP cofactor resulted in the production of 18O-enriched GP, in which 18O enrichment was reduced from ≈28 % to ≈13 % by conversion (Figure 2a). This result indicated that the acyl ligand of the FeGP cofactor is enriched with 18O, and the decrease in 18O occupancy in GP after photolysis suggested that a substantial amount of the 18O-labeled acyl group of the FeGP cofactor was scrambled with the CO groups during biosynthesis and/or photolysis.
MS/MS spectra of the FeGP cofactor show two signals, namely, proximal (607 m/z) and distal (551 m/z) signals in addition to the parent signal (635 m/z) (Figure 2d). The parent signal corresponds to the FeGP cofactor lacking the external ligands weakly bound to the iron center. The proximal MS/MS signal corresponds to the FeGP cofactor that lacks one CO or one acyl ligand because the mass difference (28 m/z) from the parent signal is equal to the mass of either the CO or acyl ligand. The distal signal corresponds to a species of the FeGP cofactor that lost all CO and acyl ligands.
By in vitro biosynthesis of the FeGP cofactor using nonlabelled GP under 50 % H2/50 % [13C]-CO, up to two molecules of [13C]-CO were incorporated into the cofactor (Figure 2c).8 Light decomposition of the labeled cofactor yielded GP, of which ≈10 % of the GP molecules were enriched with one 13C.8 In this study, we further analyzed this phenomenon by MS/MS. The MS/MS signal of the [13C1]-labeled FeGP cofactor species (636 m/z) indicated the presence of two proximal MS/MS signals at 608.03 and 607.03 m/z (Figure 2e), which correspond to dissociation of one [12C]-CO and one [13C]-CO unit from the parent species, respectively (Figure 2e). The MS/MS signal of the [13C2]-labeled FeGP cofactor (637 m/z) again indicated the presence of the two proximal MS/MS signals observed in the signal from the [13C1]-labeled species (Figure 2f). These results were in accordance with the hypothesis that the proximal MS/MS signal results from the dissociation of a CO ligand or the acyl ligand.
By performing MS/MS with the aforementioned [18O1]-labeled FeGP cofactor, we estimated the nature of the dissociated ligand, which leads to the formation of the proximal signal. The MS/MS spectrum for the [18O1]-labeled FeGP cofactor signal (637 m/z) exhibited only one proximal signal at 609 m/z, which corresponds to a species lacking one [16O]-CO unit from the intact cofactor (Figure 2b). This result indicated that the proximal 609 m/z signal resulted from the dissociation of a [16O]-CO ligand rather than an acyl ligand because the acyl ligand should exhibit some enrichment with 18O. This result suggested that the 18O-labeled acyl ligand of the FeGP cofactor is not scrambled with the CO ligand during biosynthesis.
To examine the scrambling of the CO and acyl ligands upon photolysis, we performed time-resolved infrared difference spectroscopy (Figure 3, Figure S2). Negative bands in the difference spectra represent signals from the species in the “dark” state before photoactivation takes place, while positive bands represent signals from the products after photolysis (Figure 3). The change in the infrared spectra can be classified roughly into two groups according to dissociation kinetics. The first change occurs after UV-light illumination for up to 30 seconds. During this period, two peaks at 2016 and 1953 cm−1, assigned to the original two CO ligands, decrease with time, where the rate of decrease gradually increased (Figure 4a). Concomitant with this, three peaks transiently appear at 2067, 1990, and 1925 cm−1. The infrared frequencies of the three possible CO bands are similar to those of CO-inhibited [Fe]-hydrogenase (2074, 2020 and 1981 cm−1).2b Based on the published calculation result of the infrared bands2b and the geometry of the active site of [Fe]-hydrogenase,2g we can predict that the three CO ligands bind to iron with angles between the three CO ligands of nearly 90° in the transient acyl-Fe(II)(CO)3 intermediate (Figure 4b). This finding suggests that the acyl and CO ligands are released as a CO molecule by a rapid photolysis reaction (Figure 4b, Step 1) and that the dissociated CO is ligated to an intact cofactor in the sample (Figure 4b, Step 2).
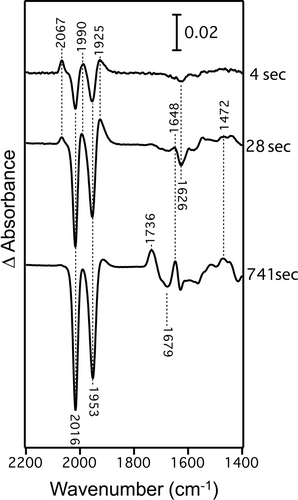
Time-resolved infrared difference spectra of the FeGP cofactor during photolysis at 4 sec, 28 sec and 741 sec after UV-light irradiation (370 nm).
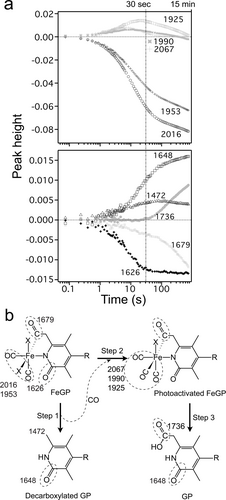
Analysis of the photolysis of the FeGP cofactor. (a) Kinetics of the infrared band intensities. The corresponding peak positions of the bands are shown. (b) Proposed mechanism for the photolysis of the FeGP cofactor. R: GMP moiety. X: Fe coordination sites weakly bound with an external stabilizing reagent and/or water. Infrared frequency (cm−1) of the parts indicated by dashed circle is shown. The detected infrared band frequencies in panel a are shown. By photoactivation, the acyl ligand is dissociated as CO and then the CO ligands also dissociate (step1). The three released CO molecules diffuse and bind to the intact cofactors in the sample to form the acyl-Fe(CO)3 intermediate (Step 2). Appearance of the carboxy group (1736 cm−1) starts after saturation of the intermediate (1990, 1925 and 2067 cm−1) (Step 3).
Accompanying the increase in these three CO bands, we observed an increase in a band at ≈1472 cm−1. From the broad band features, we assigned this band to the out-of-phase deformation mode of the CH3 group in decarboxylated GP as a product from decomposition of the acyl C=O ligand (Figure 4b, Step 2). The dissociation of the acyl group was observed as a peak at 1679 cm−1, which decreases the intensity in similar rate as the increase in the CH3 band at 1472 cm−1. Concomitant with these spectral changes, we observed decreasing (1626 cm−1) and increasing (1648 cm−1) band features that overlapped in a narrow spectral range. These observed stretches at 1626 cm−1 and 1648 cm−1 emanate from the formal and localized C=O of the deprotonated pyridone unit of the pyridone ring ligated to iron (1626 cm−1) and the iron-free pyridone after decomposition.17
Three CO peaks start to decrease after 30 seconds and completely disappear after ≈15 minutes. This result suggests that decomposition of the acyl-Fe(CO)3 state starts after 30 seconds. Accompanying the decrease in the acyl-Fe(CO)3 CO bands, a new band starts to increase at 1736 cm−1. We assigned this band to the C=O stretching mode of the carboxyl group as a product of acyl-Fe(CO)3 state decomposition (Figure 4b, Step 3). With the increase in the band at 1736 cm−1, the decrease in the acyl C=O band at 1679 cm−1 is accelerated, while the increase in the CH3 band at 1472 cm−1 is suppressed, which indicates that the decarboxylated GP does not form from the acyl-Fe(CO)3 state in this period. This suggests that the Fe-ligated acyl group is oxidized to a carboxyl group by decomposition of the acyl-Fe(CO)3 complex rather than by dissociation of the acyl groups that occurs in the early stage. In addition, the Fe-free pyridone ring vibration at 1648 cm−1 slowly increases, suggesting that the photodecomposition of the Fe complex to pyridone continues.
Based on the results, we propose a photolysis mechanism, as shown in Figure 4b. The C(O)−CH2 and C(O)−Fe bonds at the acyl group are activated by light, which results in the production of a transient iron complex intermediate with two CO ligands. In the transient intermediate, the iron site of the FeGP cofactor is probably alkylated by the methylene part of the cofactor, as predicted in metal-acyl complex reactions,18 and then the intermediate is broken to decarboxylated GP to form two additional free CO molecules. We confirmed the formation of decarboxylated GP by photolysis using MS analysis (Figure S3). The CO molecules produced by decomposition of the FeGP cofactor in the initial stage are trapped by the light-activated FeGP cofactor in the sample to form an acyl-Fe(CO)3 intermediate.
When [Fe]-hydrogenase is inhibited by external CO, an acyl-Fe(CO)3 state is formed although the CO ligands do not scramble.2b Notably, an infrared spectrum similar to the acyl-Fe(II)(CO)3 intermediate is observed during light-decomposition of [Fe]-hydrogenase.2b Chen et al. reported scrambling of the acyl and CO ligands of mimic complexes containing acyl-Fe(CO)3 and acyl-Fe(CO)2(6-Me-C4H3N-2-S) structures,15 and scrambling in the latter is accelerated by UV-A light.15b The properties of the mimic compounds provide evidence that in the light-activated acyl-Fe(CO)3 intermediate of the FeGP cofactor, the acyl and CO ligands can scramble.
In this study, we confirmed that GP was incorporated into the FeGP cofactor by in vitro biosynthesis using stable isotope labeling. Using this technique, we enriched the acyl ligand of the FeGP cofactor with 18O, which demonstrates that the carboxy group of GP is the direct precursor of the acyl ligand. Based on the MS/MS analysis of the [13C]- and [18O]-labeled FeGP cofactor, we identified that the proximal MS/MS signal corresponds to a fragmented FeGP cofactor lacking one of the CO ligands, and the CO- and acyl-ligands do not scramble during biosynthesis. This finding rules out any biosynthesis mechanisms allowing acyl/CO ligands scrambling.13 Time-resolved infrared spectroscopy indicated that an acyl-Fe(CO)3 intermediate forms by photolysis of the isolated FeGP cofactor, which can allow the acyl and CO ligands in the FeGP cofactor to scramble. This finding also suggests that the light-excited FeGP cofactor has a higher affinity for external CO at room temperature. These results are important for elucidating the biosynthesis mechanism of this unique H2-activated cofactor and for future applications of the photosensitive FeGP cofactor.
Supporting Information
The authors have cited additional references within the Supporting Information (Refs. [18–20]).
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft, Priority Program, Iron-Sulfur for Life (SPP1927, SH87/1-1 and SH87/1-2) and by the Max Planck Society to S.Sh. F.J.A.-G. was supported by the International Max Planck Research School (IMPRS) in Marburg. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




